Role of Ergothioneine in Microbial Physiology and Pathogenesis
- PMID: 28791878
- PMCID: PMC5790434
- DOI: 10.1089/ars.2017.7300
Role of Ergothioneine in Microbial Physiology and Pathogenesis
Abstract
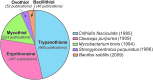
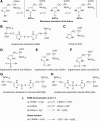
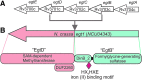
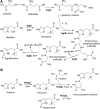
Significance: L-ergothioneine is synthesized in actinomycetes, cyanobacteria, methylobacteria, and some fungi. In contrast to other low-molecular-weight redox buffers, glutathione and mycothiol, ergothioneine is primarily present as a thione rather than a thiol at physiological pH, which makes it resistant to autoxidation. Ergothioneine regulates microbial physiology and enables the survival of microbes under stressful conditions encountered in their natural environments. In particular, ergothioneine enables pathogenic microbes, such as Mycobacterium tuberculosis (Mtb), to withstand hostile environments within the host to establish infection. Recent Advances: Ergothioneine has been reported to maintain bioenergetic homeostasis in Mtb and protect Mtb against oxidative stresses, thereby enhancing the virulence of Mtb in a mouse model. Furthermore, ergothioneine augments the resistance of Mtb to current frontline anti-TB drugs. Recently, an opportunistic fungus, Aspergillus fumigatus, which infects immunocompromised individuals, has been found to produce ergothioneine, which is important in conidial health and germination, and contributes to the fungal resistance against redox stresses.
Critical issues: The molecular mechanisms of the functions of ergothioneine in microbial physiology and pathogenesis are poorly understood. It is currently not known if ergothioneine is used in detoxification or antioxidant enzymatic pathways. As ergothioneine is involved in bioenergetic and redox homeostasis and antibiotic susceptibility of Mtb, it is of utmost importance to advance our understanding of these mechanisms.
Future directions: A clear understanding of the role of ergothioneine in microbes will advance our knowledge of how this thione enhances microbial virulence and resistance to the host's defense mechanisms to avoid complete eradication. Antioxid. Redox Signal. 28, 431-444.
Keywords: ergothioneine; microbes; oxidative stresses; redox homeostasis; thiols.
Figures



References
-
- Akanmu D, Cecchini R, Aruoma OI, and Halliwell B. The antioxidant action of ergothioneine. Arch Biochem Biophys 288: 10–16, 1991 - PubMed
-
- Aruoma OI, Whiteman M, England TG, and Halliwell B. Antioxidant action of ergothioneine: assessment of its ability to scavenge peroxynitrite. Biochem Biophys Res Commun 231: 389–391, 1997 - PubMed
-
- Av-Gay Y. and Everett M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol 8: 238–244, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
