Acceleration of Fracture Healing by Overexpression of Basic Fibroblast Growth Factor in the Mesenchymal Stromal Cells
- PMID: 28792122
- PMCID: PMC6430058
- DOI: 10.1002/sctm.17-0039
Acceleration of Fracture Healing by Overexpression of Basic Fibroblast Growth Factor in the Mesenchymal Stromal Cells
Abstract
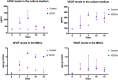
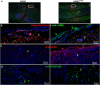
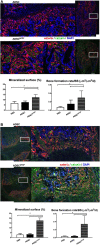
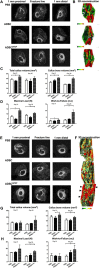
In this study, we engineered mesenchymal stem cells (MSCs) to over-express basic fibroblast growth factor (bFGF) and evaluated its effects on fracture healing. Adipose-derived mouse MSCs were transduced to express bFGF and green fluorescence protein (ADSCbFGF -GFP). Closed-femoral fractures were performed with osterix-mCherry reporter mice of both sexes. The mice received 3 × 105 ADSCs transfected with control vector or bFGF via intramuscular injection within or around the fracture sites. Mice were euthanized at days 7, 14, and 35 to monitor MSC engraftment, osteogenic differentiation, callus formation, and bone strength. Compared to ADSC culture alone, ADSCbFGF increased bFGF expression and higher levels of bFGF and vascular endothelial growth factor (VEGF) in the culture supernatant for up to 14 days. ADSCbFGF treatment increased GFP-labeled MSCs at the fracture gaps and these cells were incorporated into the newly formed callus. quantitative reverse transcription polymerase chain reaction (qRT-PCR) from the callus revealed a 2- to 12-fold increase in the expression of genes associated with nervous system regeneration, angiogenesis, and matrix formation. Compared to the control, ADSCbFGF treatment increased VEGF expression at the periosteal region of the callus, remodeling of collagen into mineralized callus and bone strength. In summary, MSCbFGF accelerated fracture healing by increasing the production of growth factors that stimulated angiogenesis and differentiation of MSCs to osteoblasts that formed new bone and accelerated fracture repair. This novel treatment may reduce the time required for fracture healing. Stem Cells Translational Medicine 2017;6:1880-1893.
Keywords: Basic fibroblast growth factor; Bone strength; Callus; Mesenchymal stromal cells; Osteoblasts.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Conflict of interest statement
The authors indicated no potential conflicts of interest.
Figures

Similar articles
-
Improved Mobilization of Exogenous Mesenchymal Stem Cells to Bone for Fracture Healing and Sex Difference.Stem Cells. 2016 Oct;34(10):2587-2600. doi: 10.1002/stem.2433. Epub 2016 Jul 15. Stem Cells. 2016. PMID: 27334693 Free PMC article.
-
Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds.Biomed Mater. 2006 Sep;1(3):93-9. doi: 10.1088/1748-6041/1/3/001. Epub 2006 Jun 5. Biomed Mater. 2006. PMID: 18458388
-
Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy.Stem Cells. 2011 Nov;29(11):1727-37. doi: 10.1002/stem.720. Stem Cells. 2011. PMID: 21898687 Free PMC article.
-
Angiogenesis after administration of basic fibroblast growth factor induces proliferation and differentiation of mesenchymal stem cells in elastic perichondrium in an in vivo model: mini review of three sequential republication-abridged reports.Cell Mol Biol Lett. 2018 Oct 5;23:49. doi: 10.1186/s11658-018-0113-1. eCollection 2018. Cell Mol Biol Lett. 2018. PMID: 30323846 Free PMC article. Review.
-
Adult mesenchymal stem cells: is there a role for purine receptors in their osteogenic differentiation?Purinergic Signal. 2020 Sep;16(3):263-287. doi: 10.1007/s11302-020-09703-4. Epub 2020 Jun 4. Purinergic Signal. 2020. PMID: 32500422 Free PMC article. Review.
Cited by
-
Strategy for the Generation of Engineered Bone Constructs Based on Umbilical Cord Mesenchymal Stromal Cells Expanded with Human Platelet Lysate.Stem Cells Int. 2019 Dec 1;2019:7198215. doi: 10.1155/2019/7198215. eCollection 2019. Stem Cells Int. 2019. PMID: 31885622 Free PMC article.
-
High Mannose N-Glycans Promote Migration of Bone-Marrow-Derived Mesenchymal Stromal Cells.Int J Mol Sci. 2020 Sep 29;21(19):7194. doi: 10.3390/ijms21197194. Int J Mol Sci. 2020. PMID: 33003435 Free PMC article.
-
New developments in the biology of fibroblast growth factors.WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review.
-
Preclinical and Clinical Amelioration of Bone Fractures with Mesenchymal Stromal Cells: a Systematic Review and Meta-Analysis.Cell Transplant. 2022 Jan-Dec;31:9636897211051743. doi: 10.1177/09636897211051743. Cell Transplant. 2022. PMID: 35916286 Free PMC article.
-
An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity.Adv Sci (Weinh). 2023 Jul;10(20):e2207334. doi: 10.1002/advs.202207334. Epub 2023 May 10. Adv Sci (Weinh). 2023. PMID: 37162248 Free PMC article. Review.
References
-
- Maciejewski ML, Radcliff TA, Henderson WG et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev 2013;50:1267–1276. - PubMed
-
- Alkhiary YM, Gerstenfeld LC, Krall E et al. Enhancement of experimental fracture‐healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am 2005;87:731–741. - PubMed
-
- Zhang D, Potty A, Vyas P et al. The role of recombinant PTH in human fracture healing: A systematic review. J Orthop Trauma 2014;28:57–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

