Thermodynamic and Transport Properties of Crown-Ethers: Force Field Development and Molecular Simulations
- PMID: 28792215
- PMCID: PMC5592649
- DOI: 10.1021/acs.jpcb.7b06547
Thermodynamic and Transport Properties of Crown-Ethers: Force Field Development and Molecular Simulations
Abstract
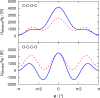
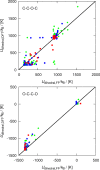
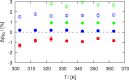
Crown-ethers have recently been used to assemble porous liquids (PLs), which are liquids with permanent porosity formed by mixing bulky solvent molecules (e.g., 15-crown-5 ether) with solvent-inaccessible organic cages. PLs and crown-ethers belong to a novel class of materials, which can potentially be used for gas separation and storage, but their performance for this purpose needs to be assessed thoroughly. Here, we use molecular simulations to study the gas separation performance of crown-ethers as the solvent of porous liquids. The TraPPE force field for linear ether molecules has been adjusted by fitting a new set of torsional potentials to accurately describe cyclic crown-ether molecules. Molecular dynamics (MD) simulations have been used to compute densities, shear viscosities, and self-diffusion coefficients of 12-crown-4, 15-crown-5, and 18-crown-6 ethers. In addition, Monte Carlo (MC) simulations have been used to compute the solubility of the gases CO2, CH4, and N2 in 12-crown-4 and 15-crown-5 ether. The computed properties are compared with available experimental data of crown-ethers and their linear counterparts, i.e., polyethylene glycol dimethyl ethers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Pedersen C. J. Cyclic Polyethers and their Complexes with Metal Salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. 10.1021/ja01002a035. - DOI
-
- Mohammadzadeh Kakhki R. Application of Crown Ethers as Stationary Phase in the Chromatographic Methods. J. Inclusion Phenom. Mol. Recognit. Chem. 2013, 75, 11–22. 10.1007/s10847-012-0158-0. - DOI
-
- Arnaud-Neu F.; Delgado R.; Chaves S. Critical Evaluation of Stability Constants and Thermodynamic Functions of Metal Complexes of Crown Ethers (IUPAC technical report). Pure Appl. Chem. 2003, 75, 71–102. 10.1351/pac200375010071. - DOI
-
- Yoshio M.; Noguchi H. Crown Ethers for Chemical Analysis: A Review. Anal. Lett. 1982, 15, 1197–1276. 10.1080/00032718208065134. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

