l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents
- PMID: 28792489
- PMCID: PMC5678004
- DOI: 10.1038/ijo.2017.164
l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents
Abstract
Objective: High-protein diets (HPDs) are associated with greater satiety and weight loss than diets rich in other macronutrients. The exact mechanisms by which HPDs exert their effects are unclear. However, evidence suggests that the sensing of amino acids produced as a result of protein digestion may have a role in appetite regulation and satiety. We investigated the effects of l-phenylalanine (L-Phe) on food intake and glucose homeostasis in rodents.
Methods: We investigated the effects of the aromatic amino-acid and calcium-sensing receptor (CaSR) agonist l-phenylalanine (L-Phe) on food intake and the release of the gastrointestinal (GI) hormones peptide YY (PYY), glucagon-like peptide-1 (GLP-1) and ghrelin in rodents, and the role of the CaSR in mediating these effects in vitro and in vivo. We also examined the effect of oral l-Phe administration on glucose tolerance in rats.
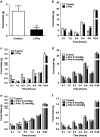
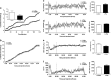
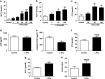
Results: Oral administration of l-Phe acutely reduced food intake in rats and mice, and chronically reduced food intake and body weight in diet-induced obese mice. Ileal l-Phe also reduced food intake in rats. l-Phe stimulated GLP-1 and PYY release, and reduced plasma ghrelin, and also stimulated insulin release and improved glucose tolerance in rats. Pharmacological blockade of the CaSR attenuated the anorectic effect of intra-ileal l-Phe in rats, and l-Phe-induced GLP-1 release from STC-1 and primary L cells was attenuated by CaSR blockade.
Conclusions: l-Phe reduced food intake, stimulated GLP-1 and PYY release, and reduced plasma ghrelin in rodents. Our data provide evidence that the anorectic effects of l-Phe are mediated via the CaSR, and suggest that l-Phe and the CaSR system in the GI tract may have therapeutic utility in the treatment of obesity and diabetes. Further work is required to determine the physiological role of the CaSR in protein sensing in the gut, and the role of this system in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Emerging Risk Factors CEmerging Risk Factors CWormser D, Emerging Risk Factors CKaptoge S, Emerging Risk Factors CDi Angelantonio E, Emerging Risk Factors CWood AM, Emerging Risk Factors CPennells L et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011; 377: 1085–1095. - PMC - PubMed
-
- Vazquez G, Duval S, Jacobs DR Jr., Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev 2007; 29: 115–128. - PubMed
-
- Poppitt SD, McCormack D, Buffenstein R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol Behav 1998; 64: 279–285. - PubMed
-
- Hochstenbach-Waelen A, Veldhorst MA, Nieuwenhuizen AG, Westerterp-Plantenga MS, Westerterp KR. Comparison of 2 diets with either 25% or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am J Clin Nutr 2009; 89: 831–838. - PubMed
-
- Wellendorph P, Johansen LD, Brauner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol 2009; 76: 453–465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

