The ProTide Prodrug Technology: From the Concept to the Clinic
- PMID: 28792763
- PMCID: PMC7075648
- DOI: 10.1021/acs.jmedchem.7b00734
The ProTide Prodrug Technology: From the Concept to the Clinic
Abstract
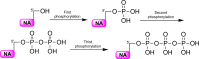
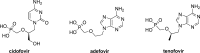
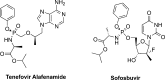
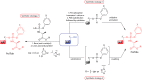
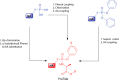
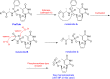
The ProTide technology is a prodrug approach developed for the efficient intracellular delivery of nucleoside analogue monophosphates and monophosphonates. In this approach, the hydroxyls of the monophosphate or monophosphonate groups are masked by an aromatic group and an amino acid ester moiety, which are enzymatically cleaved-off inside cells to release the free nucleoside monophosphate and monophosphonate species. Structurally, this represents the current end-point of an extensive medicinal chemistry endeavor that spans almost three decades. It started from the masking of nucleoside monophosphate and monophosphonate groups by simple alkyl groups and evolved into the sophisticated ProTide system as known today. This technology has been extensively employed in drug discovery, and it has already led to the discovery of two FDA-approved (antiviral) ProTides. In this work, we will review the development of the ProTide technology, its application in drug discovery, and its role in the improvement of drug delivery and efficacy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Fyfe J. A.; Keller P. M.; Furman P. A.; Miller R. L.; Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J. Biol. Chem. 1978, 253, 8721–8727. - PubMed
-
- De Clercq E. Nucleoside analogues as antiviral agents. Acta Microbiol. Acad. Sci. Hung. 1981, 28, 289–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

