Intracellular lipid droplets support osteoblast function
- PMID: 28792783
- PMCID: PMC5638385
- DOI: 10.1080/21623945.2017.1356505
Intracellular lipid droplets support osteoblast function
Abstract
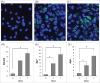
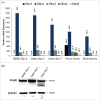
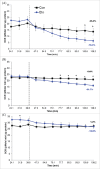
Bone formation is an osteoblast-specific process characterized by high energy demands due to the secretion of matrix proteins and mineralization vesicles. While glucose has been reported as the principle fuel source for osteoblasts, recent evidence supports the tenet that osteoblasts can utilize fatty acids as well. Although the ability to accumulate lipid droplets has been demonstrated in many cell types, there has been little evidence that osteoblasts possess this characteristic. The current study provides evidence that osteoblastogenesis is associated with lipid droplet accumulation capable of supplying energy substrates (fatty acids) required for the differentiation process. Understanding the role of fatty acids in metabolic programming of the osteoblast may lead to novel approaches to increase bone formation and ultimately bone mass.
Keywords: bone; fatty acids; lipolysis; lipophagy; marrow adipocytes.
Figures

References
-
- Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799-08. https://doi.org/10.1172/JCI117034; PMID:8113412 - DOI - PMC - PubMed
-
- Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729-39. https://doi.org/10.1002/jbmr.412; PMID:21520276 - DOI - PubMed
-
- Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:2351211-1214. PMID:13802862 - PubMed
-
- Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:2351206-1210. PMID:13802861 - PubMed
-
- Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. Biochem J. 11-15-1987;248(1):129-37. https://doi.org/10.1042/bj2480129; PMID:3325035 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
