TRPA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway
- PMID: 28792844
- PMCID: PMC5786180
- DOI: 10.1080/19336950.2017.1365206
TRPA1 ion channel stimulation enhances cardiomyocyte contractile function via a CaMKII-dependent pathway
Abstract
Rationale: Transient receptor potential channels of the ankyrin subtype-1 (TRPA1) are non-selective cation channels that show high permeability to calcium. Previous studies from our laboratory have demonstrated that TRPA1 ion channels are expressed in adult mouse ventricular cardiomyocytes (CMs) and are localized at the z-disk, costamere and intercalated disk. The functional significance of TRPA1 ion channels in the modulation of CM contractile function have not been explored.
Objective: To identify the extent to which TRPA1 ion channels are involved in modulating CM contractile function and elucidate the cellular mechanism of action.
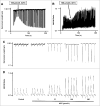
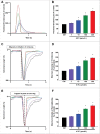
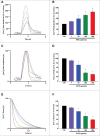
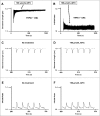
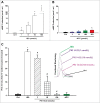
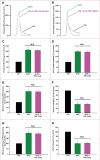
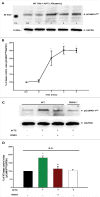
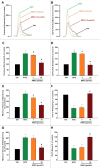
Methods and results: Freshly isolated CMs were obtained from murine heart and loaded with Fura-2 AM. Simultaneous measurement of intracellular free Ca2+ concentration ([Ca2+]i) and contractility was performed in individual CMs paced at 0.3 Hz. Our findings demonstrate that TRPA1 stimulation with AITC results in a dose-dependent increase in peak [Ca2+]i and a concomitant increase in CM fractional shortening. Further analysis revealed a dose-dependent acceleration in time to peak [Ca2+]i and velocity of shortening as well as an acceleration in [Ca2+]i decay and velocity of relengthening. These effects of TRPA1 stimulation were not observed in CMs pre-treated with the TRPA1 antagonist, HC-030031 (10 µmol/L) nor in CMs obtained from TRPA1-/- mice. Moreover, we observed no significant increase in cAMP levels or PKA activity in response to TRPA1 stimulation and the PKA inhibitor peptide (PKI 14-22; 100 nmol/L) failed to have any effect on the TRPA1-mediated increase in CM contractile function. However, TRPA1 stimulation resulted in a rapid phosphorylation of Ca2+/calmodulin-dependent kinase II (CaMKII) (1-5 min) that correlated with increases in CM [Ca2+]i and contractile function. Finally, all aspects of TRPA1-dependent increases in CM [Ca2+]i, contractile function and CaMKII phosphorylation were virtually abolished by the CaMKII inhibitors, KN-93 (10 µmol/L) and autocamtide-2-related peptide (AIP; 20 µmol/L).
Conclusions: These novel findings demonstrate that stimulation of TRPA1 ion channels in CMs results in activation of a CaMKII-dependent signaling pathway resulting in modulation of intracellular Ca2+ availability and handling leading to increases in CM contractile function. Cardiac TRPA1 ion channels may represent a novel therapeutic target for increasing the inotropic and lusitropic state of the heart.
Keywords: CaMKII; TRPA1; [Ca2+]i; cardiomyocytes; contractility.
Figures
References
-
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al.. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperature. Cell. 2003;112:819-29. doi: 10.1016/S0092-8674(03)00158-2. PMID:12654248 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
