Everolimus long-term use in patients with tuberous sclerosis complex: Four-year update of the EXIST-2 study
- PMID: 28792952
- PMCID: PMC5549893
- DOI: 10.1371/journal.pone.0180939
Everolimus long-term use in patients with tuberous sclerosis complex: Four-year update of the EXIST-2 study
Abstract
Objectives: We examined the long-term effects of everolimus in patients with renal angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis.
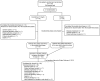
Methods: Following favorable results from the double-blind core phase of EXIST-2 (NCT00790400), patients were allowed to receive open-label everolimus (extension phase). Patients initially randomly assigned to everolimus continued on the same dose; those who were receiving placebo crossed over to everolimus 10 mg/day. Dose modifications were based on tolerability. The primary end point was angiomyolipoma response rate, defined as a ≥50% reduction from baseline in the sum volume of target renal angiomyolipomas in the absence of new target angiomyolipomas, kidney volume increase of >20% from nadir, and angiomyolipoma-related bleeding grade ≥2. The key secondary end point was safety.
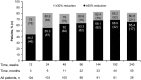
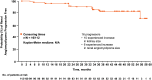
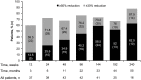
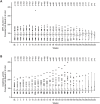
Results: Of the 112 patients who received ≥1 dose of everolimus, 58% (95% CI, 48.3% to 67.3%) achieved angiomyolipoma response. Almost all patients (97%) experienced reduction in renal lesion volumes at some point during the study period. Median duration of everolimus exposure was 46.9 months. Sixteen (14.3%) patients experienced angiomyolipoma progression at some point in the study. No angiomyolipoma-related bleeding or nephrectomies were reported. One patient on everolimus underwent embolization for worsening right flank pain. Subependymal giant cell astrocytoma lesion response was achieved in 48% of patients and skin lesion response in 68% of patients. The most common adverse events suspected to be treatment-related were stomatitis (42%), hypercholesterolemia (30.4%), acne (25.9%), aphthous stomatitis and nasopharyngitis (each 21.4%). Ten (8.9%) patients withdrew because of an adverse event. Renal function remained stable, and the frequency of emergent adverse events generally decreased over time.
Conclusions: Everolimus treatment remained safe and effective over approximately 4 years. The overall risk/benefit assessment supports the use of everolimus as a viable treatment option for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis.
Trial registration: ClinicalTrials.gov NCT00790400.
Conflict of interest statement
Figures

References
-
- Osborne JP, Fryer A, Webb D: Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991; 615: 125–127. - PubMed
-
- Curatolo P, Bombardieri R, Jozwiak S: Tuberous sclerosis. Lancet. 2008; 372: 657–668. doi: 10.1016/S0140-6736(08)61279-9 - DOI - PubMed
-
- Crino PB, Nathanson KL, Henske EP: The tuberous sclerosis complex. N Engl J Med. 2006; 355: 1345–1356. doi: 10.1056/NEJMra055323 - DOI - PubMed
-
- Budde K, Gaedeke J: Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis. 2012; 59: 276–283. doi: 10.1053/j.ajkd.2011.10.013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

