Topical Sinecatechins, 10%, Ointment for Superficial Basal Cell Carcinoma: A Randomized Clinical Trial
- PMID: 28793140
- PMCID: PMC5817560
- DOI: 10.1001/jamadermatol.2017.2529
Topical Sinecatechins, 10%, Ointment for Superficial Basal Cell Carcinoma: A Randomized Clinical Trial
Abstract
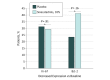
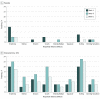
This randomized clinical trial compares topical sinecatechins ointment, 10%, vs placebo in the treatment of superficial basal cell carcinoma.
Conflict of interest statement
Figures
References
-
- Roop D, Toftgård R. Hedgehog in Wnterland. Nat Genet. 2008;40(9):1040-1041. - PubMed
-
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5(12):997-1014. - PubMed
-
- Vidal D, Matías-Guiu X, Alomar A. Efficacy of imiquimod for the expression of Bcl-2, Ki67, p53 and basal cell carcinoma apoptosis. Br J Dermatol. 2004;151(3):656-662. - PubMed
-
- Brinkhuizen T, Frencken KJ, Nelemans PJ, et al. . The effect of topical diclofenac 3% and calcitriol 3 μg/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J Am Acad Dermatol. 2016;75(1):126-134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

