Characterizing Expression and Processing of Precursor and Mature Human tRNAs by Hydro-tRNAseq and PAR-CLIP
- PMID: 28793268
- PMCID: PMC5564215
- DOI: 10.1016/j.celrep.2017.07.029
Characterizing Expression and Processing of Precursor and Mature Human tRNAs by Hydro-tRNAseq and PAR-CLIP
Abstract
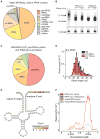
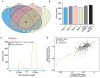
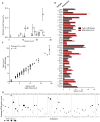
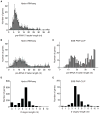
The participation of tRNAs in fundamental aspects of biology and disease necessitates an accurate, experimentally confirmed annotation of tRNA genes and curation of tRNA sequences. This has been challenging because RNA secondary structure, nucleotide modifications, and tRNA gene multiplicity complicate sequencing and mapping efforts. To address these issues, we developed hydro-tRNAseq, a method based on partial alkaline RNA hydrolysis that generates fragments amenable for sequencing. To identify transcribed tRNA genes, we further complemented this approach with photoactivatable crosslinking and immunoprecipitation (PAR-CLIP) of SSB/La, a conserved protein involved in pre-tRNA processing. Our results show that approximately half of all predicted tRNA genes are transcribed in human cells. We also report nucleotide modification sites and their order of introduction, and we identify tRNA leaders, trailers, and introns. By using complementary sequencing-based methodologies, we present a human tRNA atlas and determine expression levels of mature and processing intermediates of tRNAs in human cells.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

