Removal of Congo Red from Aqueous Solution by Anion Exchange Membrane (EBTAC): Adsorption Kinetics and Themodynamics
- PMID: 28793430
- PMCID: PMC5455645
- DOI: 10.3390/ma8074147
Removal of Congo Red from Aqueous Solution by Anion Exchange Membrane (EBTAC): Adsorption Kinetics and Themodynamics
Abstract
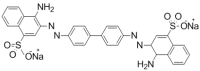
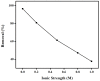
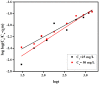
The adsorption behavior of anionic dye congo red (CR) from aqueous solutions using an anion exchange membrane (EBTAC) has been investigated at room temperature. The effect of several factors including contact time, membrane dosage, ionic strength and temperature were studied. Kinetic models, namely pseudo-first-order and pseudo-second-order, liquid film diffusion and Elovich models as well as Bangham and modified freundlich Equations, were employed to evaluate the experimental results. Parameters such as adsorption capacities, rate constant and related correlation coefficients for every model were calculated and discussed. The adsorption of CR on anion exchange membranes followed pseudo-second-order Kinetics. Thermodynamic parameters, namely changes in Gibbs free energy (∆G°), enthalpy (∆H°) and entropy (∆S°) were calculated for the adsorption of congo red, indicating an exothermic process.
Keywords: Kinetics; adsorption; anion exchange membrane; congo red; thermodynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Buckley C.A. Membrane technology for treatment of dye house effluents. Water Sci. Technol. 1992;25:203–209.
-
- Cooper P. Removing colour from dye house waste water-a critical review of technology available. J. Soc. Dyers Colour. 1993;109:97–100.
-
- Jiraratananon R., Sungpet A., Luangsowan P. Performance evaluation of nanofiltration membranes for treatment of effluents containing reactive dyes and salts. Desalination. 2000;130:177–183. doi: 10.1016/S0011-9164(00)00085-0. - DOI
-
- Karcher S., Kornmuller A., Jekel M. Screening of commercial sorbents for the removal of reactive dyes. Dyes Pigments. 2001;51:111–125. doi: 10.1016/S0143-7208(01)00066-3. - DOI
-
- Koyuncu I. Reactive dye removal in dye/salt mixtures by nanofiltration membranes containing vinylsulphone dyes: Effects of feed concentration and cross flow velocity. Desalination. 2002;143:243–253. doi: 10.1016/S0011-9164(02)00263-1. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

