Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications
- PMID: 28793602
- PMCID: PMC5455374
- DOI: 10.3390/ma8105343
Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications
Abstract
Aerogels are synthetic porous materials derived from sol-gel materials in which the liquid component has been replaced with gas to leave intact solid nanostructures without pore collapse. Recently, aerogels based on natural or synthetic polymers, called polymer or organic aerogels, have been widely explored due to their porous structures and unique properties, such as high specific surface area, low density, low thermal conductivity and dielectric constant. This paper gives a comprehensive review about the most recent progresses in preparation, structures and properties of polymer and their derived carbon-based aerogels, as well as their potential applications in various fields including energy storage, adsorption, thermal insulation and flame retardancy. To facilitate further research and development, the technical challenges are discussed, and several future research directions are also suggested in this review.
Keywords: carbon aerogels; hybrids; polymer aerogels; porous; three-dimensional network.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

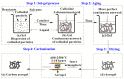

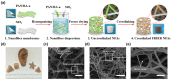






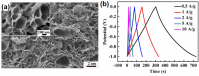
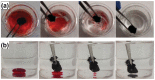


References
-
- Burger T., Fricke J. Aerogels: Production, modification and applications. Phys. Chem. Chem. Phys. 1998;102:1523–1528. doi: 10.1002/bbpc.19981021102. - DOI
-
- Fricke J., Emmerling A. Aerogels—Preparation, properties, applications. Struct. Bond. 1992;77:37–87.
-
- Cuce E., Cuce P.M., Wood C.J., Riffat S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sust. Energy Rev. 2014;34:273–299. doi: 10.1016/j.rser.2014.03.017. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

