Chitosan-Coated Collagen Membranes Promote Chondrocyte Adhesion, Growth, and Interleukin-6 Secretion
- PMID: 28793669
- PMCID: PMC5458886
- DOI: 10.3390/ma8115413
Chitosan-Coated Collagen Membranes Promote Chondrocyte Adhesion, Growth, and Interleukin-6 Secretion
Abstract
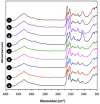
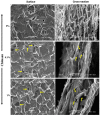
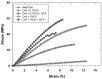
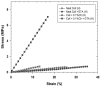
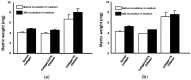
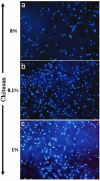
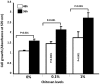
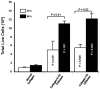
Designing scaffolds made from natural polymers may be highly attractive for tissue engineering strategies. We sought to produce and characterize chitosan-coated collagen membranes and to assess their efficacy in promoting chondrocyte adhesion, growth, and cytokine secretion. Porous collagen membranes were placed in chitosan solutions then crosslinked with glutaraldehyde vapor. Fourier transform infrared (FTIR) analyses showed elevated absorption at 1655 cm-1 of the carbon-nitrogen (N=C) bonds formed by the reaction between the (NH₂) of the chitosan and the (C=O) of the glutaraldehyde. A significant peak in the amide II region revealed a significant deacetylation of the chitosan. Scanning electron microscopy (SEM) images of the chitosan-coated membranes exhibited surface variations, with pore size ranging from 20 to 50 µm. X-ray photoelectron spectroscopy (XPS) revealed a decreased C-C groups and an increased C-N/C-O groups due to the reaction between the carbon from the collagen and the NH2 from the chitosan. Increased rigidity of these membranes was also observed when comparing the chitosan-coated and uncoated membranes at dried conditions. However, under wet conditions, the chitosan coated collagen membranes showed lower rigidity as compared to dried conditions. Of great interest, the glutaraldehyde-crosslinked chitosan-coated collagen membranes promoted chondrocyte adhesion, growth, and interleukin (IL)-6 secretion. Overall results confirm the feasibility of using designed chitosan-coated collagen membranes in future applications, such as cartilage repair.
Keywords: chitosan; chondrocytes; collagen; interleukin (IL)-6; membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The effect of type II collagen coating of chitosan fibrous scaffolds on mesenchymal stem cell adhesion and chondrogenesis.Acta Biomater. 2010 Oct;6(10):3988-97. doi: 10.1016/j.actbio.2010.05.016. Epub 2010 May 23. Acta Biomater. 2010. PMID: 20580951
-
Chitosan/γ-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly-l-lysine for cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2017 Sep 1;78:265-277. doi: 10.1016/j.msec.2017.04.067. Epub 2017 Apr 13. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28575984
-
Effect of collagen II coating on mesenchymal stem cell adhesion on chitosan and on reacetylated chitosan fibrous scaffolds.J Mater Sci Mater Med. 2010 Aug;21(8):2479-90. doi: 10.1007/s10856-010-4096-3. Epub 2010 May 25. J Mater Sci Mater Med. 2010. PMID: 20499139
-
Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts.Colloids Surf B Biointerfaces. 2011 Feb 1;82(2):307-15. doi: 10.1016/j.colsurfb.2010.09.002. Epub 2010 Sep 15. Colloids Surf B Biointerfaces. 2011. PMID: 20888196
-
3D Printed Chitosan Composite Scaffold for Chondrocytes Differentiation.Curr Med Imaging. 2021;17(7):832-842. doi: 10.2174/1573405616666201217112939. Curr Med Imaging. 2021. PMID: 33334294
Cited by
-
Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial.Polymers (Basel). 2021 Jul 26;13(15):2454. doi: 10.3390/polym13152454. Polymers (Basel). 2021. PMID: 34372060 Free PMC article.
-
Effect of Periplaneta americana Residue Feed on Immunity, Antioxidant Capacity, and Transcriptome in Chickens: A Study on Sanhuang Chickens.Animals (Basel). 2025 Jan 3;15(1):94. doi: 10.3390/ani15010094. Animals (Basel). 2025. PMID: 39795037 Free PMC article.
-
Fabrication of Porous Materials from Natural/Synthetic Biopolymers and Their Composites.Materials (Basel). 2016 Dec 7;9(12):991. doi: 10.3390/ma9120991. Materials (Basel). 2016. PMID: 28774113 Free PMC article. Review.
-
A proteomics study to explore the role of adsorbed serum proteins for PC12 cell adhesion and growth on chitosan and collagen/chitosan surfaces.Regen Biomater. 2018 Oct;5(5):261-273. doi: 10.1093/rb/rby017. Epub 2018 Jul 17. Regen Biomater. 2018. PMID: 30338124 Free PMC article.
-
Development and Characterization of Functional Polylactic Acid/Chitosan Porous Scaffolds for Bone Tissue Engineering.Polymers (Basel). 2022 Nov 23;14(23):5079. doi: 10.3390/polym14235079. Polymers (Basel). 2022. PMID: 36501473 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

