Excitable Signal Transduction Networks in Directed Cell Migration
- PMID: 28793794
- PMCID: PMC5792054
- DOI: 10.1146/annurev-cellbio-100616-060739
Excitable Signal Transduction Networks in Directed Cell Migration
Abstract
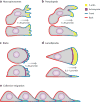
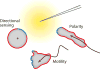
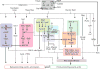
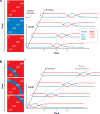
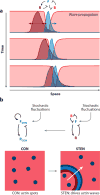
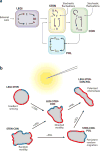
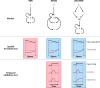
Although directed migration of eukaryotic cells may have evolved to escape nutrient depletion, it has been adopted for an extensive range of physiological events during development and in the adult organism. The subversion of these movements results in disease, such as cancer. Mechanisms of propulsion and sensing are extremely diverse, but most eukaryotic cells move by extending actin-filled protrusions termed macropinosomes, pseudopodia, or lamellipodia or by extension of blebs. In addition to motility, directed migration involves polarity and directional sensing. The hundreds of gene products involved in these processes are organized into networks of parallel and interconnected pathways. Many of these components are activated or inhibited coordinately with stimulation and on each spontaneously extended protrusion. Moreover, these networks display hallmarks of excitability, including all-or-nothing responsiveness and wave propagation. Cellular protrusions result from signal transduction waves that propagate outwardly from an origin and drive cytoskeletal activity. The range of the propagating waves and hence the size of the protrusions can be altered by lowering or raising the threshold for network activation, with larger and wider protrusions favoring gliding or oscillatory behavior over amoeboid migration. Here, we evaluate the variety of models of excitable networks controlling directed migration and outline critical tests. We also discuss the utility of this emerging view in producing cell migration and in integrating the various extrinsic cues that direct migration.
Keywords: biochemical oscillations; chemotaxis; electrotaxis; inflammation; metastasis; shear stress.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

