* Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties
- PMID: 28793839
- PMCID: PMC5729880
- DOI: 10.1089/ten.TEA.2017.0048
* Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties
Abstract
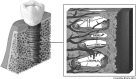
Successful dental and orthopedic implant outcomes are determined by the degree of osseointegration. Over the last 60 years, endosseous implants have evolved to stimulate osteogenesis without the need for exogenous biologics such as bone morphogenetic proteins. An understanding of the interaction between cells and the physical characteristics of their environments has led to development of bioactive implants. Implant surfaces that mimic the inherent chemistry, topography, and wettability of native bone have shown to provide cells in the osteoblast lineage with the structural cues to promote tissue regeneration and net new bone formation. Studies show that attachment, proliferation, differentiation, and local factor production are sensitive to these implant surface characteristics. This review focuses on how surface properties, including chemistry, topography, and hydrophilicity, modulate protein adsorption, cell behavior, biological reactions, and signaling pathways in peri-implant bone tissue, allowing the development of true biomimetics that promote osseointegration by providing an environment suitable for osteogenesis.
Keywords: biomimetic; implant; mesenchymal stem cell; osteoblast; osteoclast; titanium.
Conflict of interest statement
B.D.B. is an unpaid consultant for Institut Straumann AG and a paid consultant for TitanSpine LLC. Z.S. is a paid consultant for AB Dental.
Figures



References
-
- Bobbio A. The first endosseous alloplastic implant in the history of man. Bull Hist Dent 20, 1, 1972 - PubMed
-
- Branemark P.I., Hansson B.O., Adell R., Breine U., Lindstrom J., Hallen O., and Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 16, 1, 1977 - PubMed
-
- Lang B.R., and Chiappa A.A. Mandibular implants: a new method of attachment. J Prosthet Dent 22, 261, 1969 - PubMed
-
- Sykaras N., Iacopino A.M., Marker V.A., Triplett R.G., and Woody R.D. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants 15, 675, 2000 - PubMed
-
- Bencharit S., Allen R.K., and Whitley D., 3rd. Utilization of demineralized bone matrix to restore missing buccal bone during single implant placement: clinical report. J Oral Implantol 42, 490, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

