Cathepsin-S degraded decorin are elevated in fibrotic lung disorders - development and biological validation of a new serum biomarker
- PMID: 28793886
- PMCID: PMC5550991
- DOI: 10.1186/s12890-017-0455-x
Cathepsin-S degraded decorin are elevated in fibrotic lung disorders - development and biological validation of a new serum biomarker
Abstract
Background: Decorin is one of the most abundant proteoglycans of the extracellular matrix and is mainly secreted and deposited in the interstitial matrix by fibroblasts where it plays an important role in collagen turnover and tissue homeostasis. Degradation of decorin might disturb normal tissue homeostasis contributing to extracellular matrix remodeling diseases. Here, we present the development and validation of a competitive enzyme-linked immunosorbent assay (ELISA) quantifying a specific fragment of degraded decorin, which has potential as a novel non-invasive serum biomarker for fibrotic lung disorders.
Methods: A fragment of decorin cleaved in vitro using human articular cartilage was identified by mass-spectrometry (MS/MS). Monoclonal antibodies were raised against the neo-epitope of the cleaved decorin fragment and a competitive ELISA assay (DCN-CS) was developed. The assay was evaluated by determining the inter- and intra-assay precision, dilution recovery, accuracy, analyte stability and interference. Serum levels were assessed in lung cancer patients, patients with idiopathic pulmonary fibrosis (IPF), patients with chronic obstructive pulmonary disease (COPD) and healthy controls.
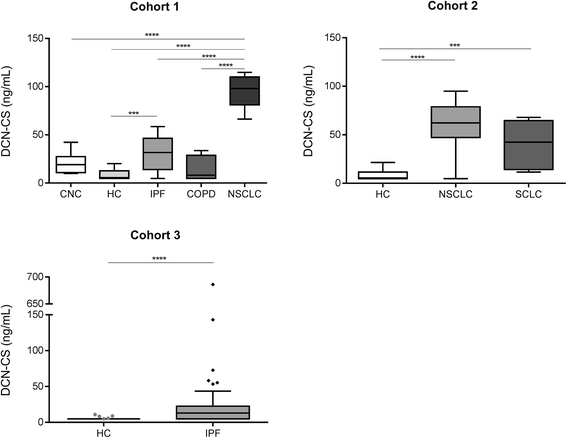
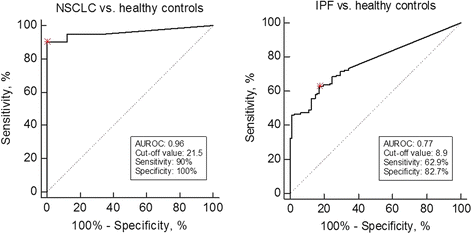
Results: The DCN-CS ELISA was technically robust and was specific for decorin cleaved by cathepsin-S. DCN-CS was elevated in lung cancer patients (p < 0.0001) and IPF patients (p < 0.001) when compared to healthy controls. The diagnostic power for differentiating lung cancer patients and IPF patients from healthy controls was 0.96 and 0.77, respectively.
Conclusion: Cathepsin-S degraded decorin could be quantified in serum using the DCN-CS competitive ELISA. The clinical data indicated that degradation of decorin by cathepsin-S is an important part of the pathology of lung cancer and IPF.
Keywords: Cancer; Cathepsin-S; Decorin; Extracellular matrix; Idiopathic pulmonary fibrosis; Serum biomarker.
Conflict of interest statement
Ethics approval and consent to participate
For the clinical IPF study (CTgov reg. NCT00786201) the following ethic committee approved the study: Sterling Institutional Review Board, Sterling Independent Services, Inc. (Atlanta). The healthy control serum samples (cohort 2) were obtained from a Danish study population approved by The National Committee on Health Research Ethics (Denmark). According to Danish law additional ethical approval for measuring biochemical biomarkers in previously collected samples is not required. For the samples obtained from the commercial vendors Proteogenex, Asterand and Valley Biomedical, appropriate Institutional Review Board/Independent Ethical Committee approved sample collection and all patients filed informed consent.
Consent for publication
Not applicable.
Competing interests
C.L. Bager, N. Willumsen, D.J. Leeming and M.A. Karsdal are employed at Nordic Bioscience A/S which is a company involved in discovery and development of biochemical biomarkers. M.A. Karsdal owns stocks at Nordic Bioscience. M. Curran, B Dasgupta and C. Brodmerkel are employees of Janssen R&D LLC. and own stock in Johnson & Johnson. S.N. Kehlet and S. Brix reports no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling.BMC Pulm Med. 2015 May 3;15:53. doi: 10.1186/s12890-015-0048-5. BMC Pulm Med. 2015. PMID: 25935650 Free PMC article.
-
Nidogen-1 Degraded by Cathepsin S can be Quantified in Serum and is Associated with Non-Small Cell Lung Cancer.Neoplasia. 2017 Apr;19(4):271-278. doi: 10.1016/j.neo.2017.01.008. Epub 2017 Mar 7. Neoplasia. 2017. PMID: 28282545 Free PMC article.
-
Type VIII collagen is elevated in diseases associated with angiogenesis and vascular remodeling.Clin Biochem. 2016 Aug;49(12):903-8. doi: 10.1016/j.clinbiochem.2016.05.023. Epub 2016 May 24. Clin Biochem. 2016. PMID: 27234597
-
Commonalities between the pro-fibrotic mechanisms in COPD and IPF.Pulm Pharmacol Ther. 2012 Aug;25(4):276-80. doi: 10.1016/j.pupt.2011.08.003. Epub 2011 Sep 29. Pulm Pharmacol Ther. 2012. PMID: 21983244 Review.
-
Generation of a multi-functional, target organ-specific, anti-fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin.Br J Pharmacol. 2019 Jan;176(1):16-25. doi: 10.1111/bph.14374. Epub 2018 Jun 25. Br J Pharmacol. 2019. PMID: 29847688 Free PMC article. Review.
Cited by
-
Leveraging 3D Model Systems to Understand Viral Interactions with the Respiratory Mucosa.Viruses. 2020 Dec 11;12(12):1425. doi: 10.3390/v12121425. Viruses. 2020. PMID: 33322395 Free PMC article. Review.
-
Proteoglycans as Immunomodulators of the Innate Immune Response to Lung Infection.J Histochem Cytochem. 2018 Apr;66(4):241-259. doi: 10.1369/0022155417751880. Epub 2018 Jan 12. J Histochem Cytochem. 2018. PMID: 29328866 Free PMC article. Review.
-
Piezo1 specific deletion in macrophage protects the progression of liver fibrosis in mice.Theranostics. 2023 Oct 2;13(15):5418-5434. doi: 10.7150/thno.86103. eCollection 2023. Theranostics. 2023. PMID: 37908726 Free PMC article.
-
Exploring the role of granzyme B in subretinal fibrosis of age-related macular degeneration.Front Immunol. 2024 Jul 18;15:1421175. doi: 10.3389/fimmu.2024.1421175. eCollection 2024. Front Immunol. 2024. PMID: 39091492 Free PMC article. Review.
-
Revisiting Circulating Extracellular Matrix Fragments as Disease Markers in Myelofibrosis and Related Neoplasms.Cancers (Basel). 2023 Aug 29;15(17):4323. doi: 10.3390/cancers15174323. Cancers (Basel). 2023. PMID: 37686599 Free PMC article. Review.
References
-
- Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen A-C, et al. Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay Drug Dev Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

