IgG Fc galactosylation predicts response to methotrexate in early rheumatoid arthritis
- PMID: 28793911
- PMCID: PMC5549282
- DOI: 10.1186/s13075-017-1389-7
IgG Fc galactosylation predicts response to methotrexate in early rheumatoid arthritis
Abstract
Background: Methotrexate (MTX) is the standard first-line therapy in rheumatoid arthritis (RA) with variable clinical efficacy that is difficult to predict. The glycosylation status of immunoglobulin G (IgG) is altered in RA and influenced by MTX treatment. We aimed to further investigate if IgG glycosylation in untreated early RA can predict therapeutic response to MTX.
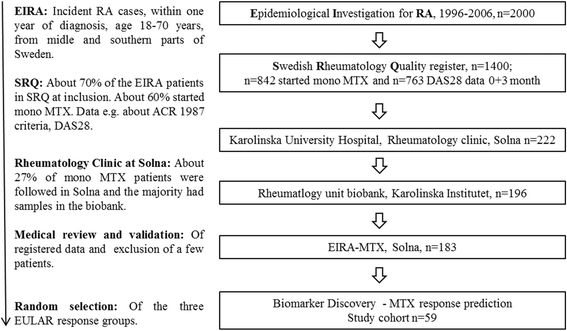
Methods: We used a shotgun proteomic approach to screen for the Fc glycopeptides in the serum of 12 control subjects and 59 untreated patients with early RA prior to and following MTX initiation. MTX treatment response was defined according to the European League Against Rheumatism at a median of 14 weeks (range 13-15) after treatment initiation. Seropositive patients were defined as those testing positive for anticitrullinated protein antibodies and/or rheumatoid factor at baseline (n = 44). Data analysis was performed using uni- and multivariate statistics.
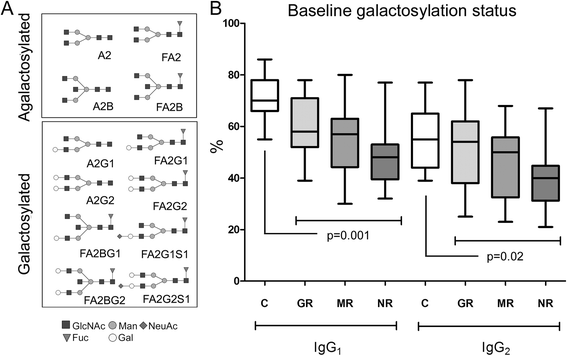
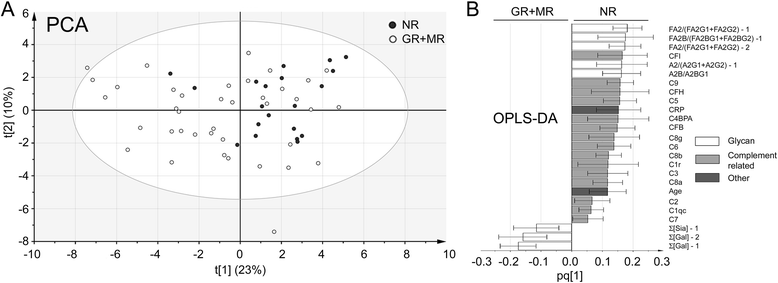
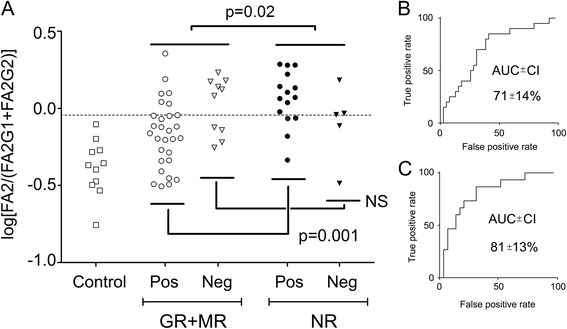
Results: We could confirm a low abundance of galactosylated glycans in untreated patients with early RA compared with control subjects that was partially restored by MTX treatment. This was more evident among future nonresponders than among responders to MTX treatment. Results were further validated and confirmed by multivariate statistical analysis of the baseline Fc glycan, proteomic, and clinical data. We found that the ratio between the main agalactosylated (FA2) and main mono- and di-galactosylated Fc glycans (FA2G1 and FA2G2) of IgG1 ranked as the most prominent factor distinguishing responders from nonresponders. A low baseline ratio of FA2/[FA2G1 + FA2G2]-IgG1 was associated with nonresponse (OR 5.3 [1.6-17.0]) and was able to discriminate future nonresponders from responders to MTX therapy with a sensitivity of 70% (95% CI 46-88%) and a specificity of 69% (95% CI 52-83%). For seropositive patients (n = 44), this trend was improved with a sensitivity of 73% (95% CI 45-92%) for nonresponse and a specificity of 79% (95% CI 60-92%).
Conclusions: We show that the FA2/[FA2G1 + FA2G2] of IgG1 is a biomarker candidate that is significantly associated with nonresponding patients and has potential value for prediction of MTX clinical response.
Keywords: Biomarker; Complement; Glycosylation; Immunoglobulin; Methotrexate; Rheumatoid arthritis.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for this work was obtained from the regional ethical review board (96-174[1996-0419] and 2006-476-31/4) and the Karolinska Institute, Stockholm, Sweden. An informed consent form for study participation and for publication of collected clinical data (as documented by caregivers in patient records), was given to all participants as specified in the ethical approval and in line with Swedish law.
Consent for publication
The participants gave their consent to use the collected clinical sample data for publication. The anonymity of the participants cannot be violated through the disclosure of the included data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

