Standardising the reporting of outcomes in gastric cancer surgery trials: protocol for the development of a core outcome set and accompanying outcome measurement instrument set (the GASTROS study)
- PMID: 28793921
- PMCID: PMC5550993
- DOI: 10.1186/s13063-017-2100-7
Standardising the reporting of outcomes in gastric cancer surgery trials: protocol for the development of a core outcome set and accompanying outcome measurement instrument set (the GASTROS study)
Abstract
Background: Gastric cancer is one of the leading causes of cancer-related deaths worldwide. Whilst surgery is the mainstay of curative treatment, it is associated with significant risks. Surgical strategies for treating gastric cancer should be based on evidence from systematic reviews of well-designed randomised controlled trials. However, inconsistencies in the reporting of outcomes from these trials makes evidence synthesis unreliable. We present a protocol for an international consensus study to develop a standardised set of outcomes and measurement tools - a 'core outcome set' (COS) - to be used by all future trials examining therapeutic surgical interventions for gastric cancer. The GASTROS study aims to standardise the reporting of outcomes in gastric cancer surgery trials through an international consensus process of key stakeholders including health care professionals and patients.
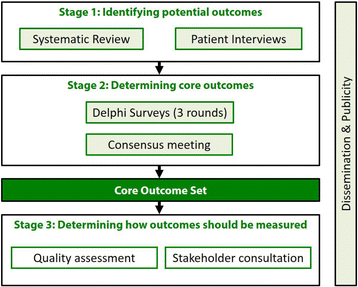
Methods: The first of three stages in the study will identify a 'long-list' of potentially important outcomes to be prioritised. These will be extracted from a systematic review of relevant academic literature and patient interviews. Stage 2 will comprise an eDelphi survey which will consider the views of patients, nurse specialists and surgeons to prioritise the most important outcomes. A meeting of stakeholder representatives will ratify the COS. Stage 3 will focus on identifying appropriate instruments to measure the prioritised outcomes by means of quality assessment of available measurement instruments and stakeholder consultation.
Discussion: This study aims to standardise the reporting of outcomes in future trials examining therapeutic surgical interventions for gastric cancer. It is anticipated that standardisation of outcome reporting in these surgical effectiveness trials will enhance the evidence base for clinical practice. Highlighting outcomes of greatest importance to patients will ensure that their perspectives are central to research in this field.
Keywords: Consensus; Outcome assessment (Health care); Patient outcome assessment; Qualitative research; Stomach neoplasms; Surgical oncology.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
This will be sought for each aspect of the study where ethical approval is required. At the time of writing, the GASTROS study is at stage 1 (Fig. 1). Ethical approval has been authorised for this stage by the NHS Health Research Authority (reference 16/ES/0151).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fact Sheets by Cancer. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 3 Feb 2016.
-
- Cancer survival for common cancers | Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/su.... Accessed 16 May 2016.
-
- Centre H & SCI. National Oesophago-Gastric Cancer Audit 2015. http://www.hscic.gov.uk/article/1165/searchcatalogue?q=%22National+Oesop.... Published 2015. Accessed 16 May 2016.
-
- Messager M, de Steur WO, van Sandick JW, et al. Variations among 5 European countries for curative treatment of resectable oesophageal and gastric cancer: a survey from the EURECCA Upper GI Group (EUropean REgistration of Cancer CAre) Eur J Surg Oncol. 2016;42(1):116–22. doi: 10.1016/j.ejso.2015.09.017. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical