Potent In Vivo NK Cell-Mediated Elimination of HIV-1-Infected Cells Mobilized by a gp120-Bispecific and Hexavalent Broadly Neutralizing Fusion Protein
- PMID: 28794022
- PMCID: PMC5625480
- DOI: 10.1128/JVI.00937-17
Potent In Vivo NK Cell-Mediated Elimination of HIV-1-Infected Cells Mobilized by a gp120-Bispecific and Hexavalent Broadly Neutralizing Fusion Protein
Abstract
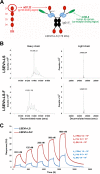
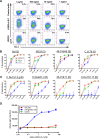
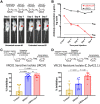
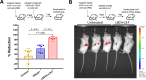
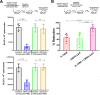
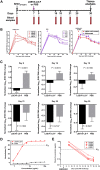
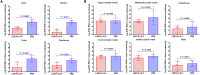
Antibodies bound to human immunodeficiency virus type 1 (HIV-1) envelope protein expressed by infected cells mobilize antibody-dependent cellular cytotoxicity (ADCC) to eliminate the HIV-1-infected cells and thereby suppress HIV-1 infection and delay disease progression. Studies treating HIV-1-infected individuals with latency reactivation agents to reduce their latent HIV-1 reservoirs indicated that their HIV-1-specific immune responses were insufficient to effectively eliminate the reactivated latent HIV-1-infected T cells. Mobilization of ADCC may facilitate elimination of reactivated latent HIV-1-infected cells to deplete the HIV-1 reservoir and contribute to a functional HIV-1 cure. The most effective antibodies for controlling and eradicating HIV-1 infection would likely have the dual capacities of potently neutralizing a broad range of HIV-1 isolates and effectively mobilizing HIV-1-specific ADCC to eliminate HIV-1-infected cells. For this purpose, we constructed LSEVh-LS-F, a broadly neutralizing, defucosylated hexavalent fusion protein specific for both the CD4 and coreceptor gp120-binding sites. LSEVh-LS-F potently inhibited in vivo HIV-1 and simian-human immunodeficiency virus (SHIV) infection in humanized mouse and macaque models, respectively, including in vivo neutralization of HIV-1 strains resistant to the broadly neutralizing antibodies VRC01 and 3BNC117. We developed a novel humanized mouse model to evaluate in vivo human NK cell-mediated elimination of HIV-1-infected cells by ADCC and utilized it to demonstrate that LSEVh-LS-F rapidly mobilized NK cells to eliminate >80% of HIV-1-infected cells in vivo 1 day after its administration. The capacity of LSEVh-LS-F to eliminate HIV-1-infected cells via ADCC combined with its broad neutralization activity supports its potential use as an immunotherapeutic agent to eliminate reactivated latent cells and deplete the HIV-1 reservoir.IMPORTANCE Mobilization of antibody-dependent cellular cytotoxicity (ADCC) to eliminate reactivated latent HIV-1-infected cells is a strategy which may contribute to depleting the HIV-1 reservoir and achieving a functional HIV-1 cure. To more effectively mobilize ADCC, we designed and constructed LSEVh-LS-F, a broadly neutralizing, defucosylated hexavalent fusion protein specific for both the CD4 and coreceptor gp120-binding sites. LSEVh-LS-F potently inhibited in vivo HIV-1 and SHIV infection in humanized mouse and macaque models, respectively, including in vivo neutralization of an HIV-1 strain resistant to the broadly neutralizing antibodies VRC01 and 3BNC117. Using a novel humanized mouse model, we demonstrated that LSEVh-LS-F rapidly mobilized NK cells to eliminate >80% of HIV-1-infected cells in vivo 1 day after its administration. The capacity of LSEVh-LS-F to eliminate HIV-1-infected cells via ADCC combined with its broad neutralization activity supports its potential use as an immunotherapeutic agent to eliminate reactivated latent cells and deplete the HIV-1 reservoir.
Keywords: NK cell; human immunodeficiency virus.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. - DOI - PMC - PubMed
-
- Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Buning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. doi: 10.1073/pnas.1315295110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

