Recombinant Origins of Pathogenic and Nonpathogenic Mouse Gammaretroviruses with Polytropic Host Range
- PMID: 28794032
- PMCID: PMC5640873
- DOI: 10.1128/JVI.00855-17
Recombinant Origins of Pathogenic and Nonpathogenic Mouse Gammaretroviruses with Polytropic Host Range
Abstract
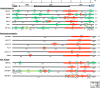
Ecotropic, xenotropic, and polytropic mouse leukemia viruses (E-, X-, and P-MLVs) exist in mice as infectious viruses and endogenous retroviruses (ERVs) inserted into mouse chromosomes. All three MLV subgroups are linked to leukemogenesis, which involves generation of recombinants with polytropic host range. Although P-MLVs are deemed to be the proximal agents of disease induction, few biologically characterized infectious P-MLVs have been sequenced for comparative analysis. We analyzed the complete genomes of 16 naturally occurring infectious P-MLVs, 12 of which were typed for pathogenic potential. We sought to identify ERV progenitors, recombinational hot spots, and segments that are always replaced, never replaced, or linked to pathogenesis or host range. Each P-MLV has an E-MLV backbone with P- or X-ERV replacements that together cover 100% of the recombinant genomes, with different substitution patterns for X- and P-ERVs. Two segments are always replaced, both coding for envelope (Env) protein segments: the N terminus of the surface subunit and the cytoplasmic tail R peptide. Viral gag gene replacements are influenced by host restriction genes Fv1 and Apobec3 Pathogenic potential maps to the env transmembrane subunit segment encoding the N-heptad repeat (HR1). Molecular dynamics simulations identified three novel interdomain salt bridges in the lymphomagenic virus HR1 that could affect structural stability, entry or sensitivity to host immune responses. The long terminal repeats of lymphomagenic P-MLVs are differentially altered by recombinations, duplications, or mutations. This analysis of the naturally occurring, sometimes pathogenic P-MLV recombinants defines the limits and extent of intersubgroup recombination and identifies specific sequence changes linked to pathogenesis and host interactions.IMPORTANCE During virus-induced leukemogenesis, ecotropic mouse leukemia viruses (MLVs) recombine with nonecotropic endogenous retroviruses (ERVs) to produce polytropic MLVs (P-MLVs). Analysis of 16 P-MLV genomes identified two segments consistently replaced: one at the envelope N terminus that alters receptor choice and one in the R peptide at the envelope C terminus, which is removed during virus assembly. Genome-wide analysis shows that nonecotropic replacements in the progenitor ecotropic MLV genome are more extensive than previously appreciated, covering 100% of the genome; contributions from xenotropic and polytropic ERVs differentially alter the regions responsible for receptor determination or subject to APOBEC3 and Fv1 restriction. All pathogenic viruses had modifications in the regulatory elements in their long terminal repeats and differed in a helical segment of envelope involved in entry and targeted by the host immune system. Virus-induced leukemogenesis thus involves generation of complex recombinants, and specific replacements are linked to pathogenesis and host restrictions.
Keywords: origins of infectious gammaretroviruses; pathogenic mouse gammaretroviruses; polytropic mouse gammaretroviruses; recombinant retroviruses; retroviral N-heptad repeat.
Copyright © 2017 American Society for Microbiology.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

