Modulation of taste processing by temperature
- PMID: 28794101
- PMCID: PMC5668616
- DOI: 10.1152/ajpregu.00089.2017
Modulation of taste processing by temperature
Abstract
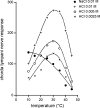
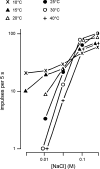
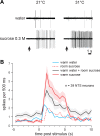
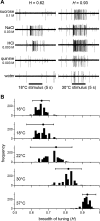
Taste stimuli have a temperature that can stimulate thermosensitive neural machinery in the mouth during gustatory experience. Although taste and oral temperature are sometimes discussed as different oral sensory modalities, there is a body of literature that demonstrates temperature is an important component and modulator of the intensity of gustatory neural and perceptual responses. Available data indicate that the influence of temperature on taste, herein referred to as "thermogustation," can vary across taste qualities, can also vary among stimuli presumed to share a common taste quality, and is conditioned on taste stimulus concentration, with neuronal and psychophysical data revealing larger modulatory effects of temperature on gustatory responding to weakened taste solutions compared with concentrated. What is more, thermogustation is evidenced to involve interplay between mouth and stimulus temperature. Given these and other dependencies, identifying principles by which thermal input affects gustatory information flow in the nervous system may be important for ultimately unravelling the organization of neural circuits for taste and defining their involvement with multisensory processing related to flavor. Yet thermal effects are relatively understudied in gustatory neuroscience. Major gaps in our understanding of the mechanisms and consequences of thermogustation include delineating supporting receptors, the potential involvement of oral thermal and somatosensory trigeminal neurons in thermogustatory interactions, and the broader operational roles of temperature in gustatory processing. This review will discuss these and other issues in the context of the literature relevant to understanding thermogustation.
Keywords: flavor; multisensory; somatosensation; taste; temperature.
Copyright © 2017 the American Physiological Society.
Figures


References
-
- Abbott P. The Effect of Temperature on Taste in the White Rat (PhD Thesis) Brown University, 1953.
-
- Afroz A, Howlett N, Shukla A, Ahmad F, Batista E, Bedard K, Payne S, Morton B, Mansfield JH, Glendinning JI. Gustatory receptor neurons in Manduca sexta contain a TrpA1-dependent signaling pathway that integrates taste and temperature. Chem Senses 38: 605–617, 2013. doi: 10.1093/chemse/bjt032. - DOI - PMC - PubMed
-
- Arai T, Ohkuri T, Yasumatsu K, Kaga T, Ninomiya Y. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience 165: 1476–1489, 2010. doi: 10.1016/j.neuroscience.2009.11.051. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

