Denervated Myocardium Is Preferentially Associated With Sudden Cardiac Arrest in Ischemic Cardiomyopathy: A Pilot Competing Risks Analysis of Cause-Specific Mortality
- PMID: 28794139
- PMCID: PMC5555076
- DOI: 10.1161/CIRCIMAGING.117.006446
Denervated Myocardium Is Preferentially Associated With Sudden Cardiac Arrest in Ischemic Cardiomyopathy: A Pilot Competing Risks Analysis of Cause-Specific Mortality
Abstract
Background: Previous studies have identified multiple risk factors that are associated with total cardiac mortality. Nevertheless, identifying specific factors that distinguish patients at risk of arrhythmic death versus heart failure could better target patients likely to benefit from implantable cardiac defibrillators, which have no impact on nonsudden cardiac death.
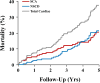
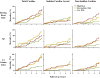
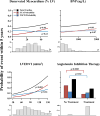
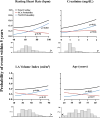
Methods and results: We performed a pilot competing risks analysis of the National Institutes of Health-sponsored PAREPET trial (Prediction of Arrhythmic Events with Positron Emission Tomography). Death from cardiac causes was ascertained in subjects with ischemic cardiomyopathy (n=204) eligible for an implantable cardiac defibrillator for the primary prevention of sudden cardiac arrest after baseline clinical evaluation and imaging at enrollment (positron emission tomography and 2-dimensional echo). Mean age was 67±11 years with an ejection fraction of 27±9%, and 90% were men. During 4.1 years of follow-up, there were 33 sudden cardiac arrests (arrhythmic death or implantable cardiac defibrillator discharge for ventricular fibrillation or ventricular tachycardia >240 bpm) and 36 nonsudden cardiac deaths. Sudden cardiac arrest was correlated with a greater volume of denervated myocardium (defect of the positron emission tomography norepinephrine analog 11C-hydroxyephedrine), lack of angiotensin inhibition therapy, elevated B-type natriuretic peptide, and larger left ventricular end-diastolic volume index. In contrast, nonsudden cardiac death was associated with a higher resting heart rate, older age, elevated creatinine, larger left atrial volume index, and larger left ventricular end-diastolic volume index.
Conclusions: Distinct clinical, laboratory, and imaging variables are associated with cause-specific cardiac mortality in primary-prevention candidates with ischemic cardiomyopathy. If prospectively validated, these multivariable associations may help target specific therapies to those at the greatest risk of sudden and nonsudden cardiac death.
Clinical trial registration: URL: https://clinicaltrials.gov. Unique identifier: NCT01400334.
Keywords: coronary artery disease; death, sudden, cardiac; defibrillators, implantable; heart failure; positron-emission tomography; risk factors.
© 2017 American Heart Association, Inc.
Figures


References
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. - PubMed
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. - PubMed
-
- Berger R, Huelsman M, Strecker K, Bojic A, Moser P, Stanek B, Pacher R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. - PubMed
-
- Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole-Wilson PA, Levy WC. Prediction of mode of death in heart failure: The seattle heart failure model. Circulation. 2007;116:392–398. - PubMed
-
- Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

