Tubulointerstitial Nephritis with IgM-Positive Plasma Cells
- PMID: 28794148
- PMCID: PMC5698061
- DOI: 10.1681/ASN.2016101074
Tubulointerstitial Nephritis with IgM-Positive Plasma Cells
Abstract
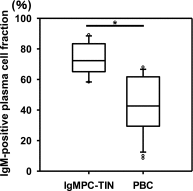
Infiltration by IgG-positive plasma cells is a common finding in tubulointerstitial nephritis. Indeed, it has been thought that CD138-positive mature plasma cells secrete mainly IgG, and the occurrence of tubulointerstitial nephritis with CD138-positive plasma cells secreting IgM has rarely been reported. Routine immunofluorescence of fresh frozen sections is considered the gold standard for detection of immune deposits. However, the immunoenzyme method with formalin-fixed, paraffin-embedded sections is superior for detecting IgM- or IgG-positive cells within the renal interstitium, thus histologic variants may often go undetected. We recently discovered a case of tubulointerstitial nephritis showing IgM-positive plasma cell accumulation within the interstitium. To further explore the morphologic and clinical features of such cases, we performed a nationwide search for patients with biopsy-proven tubulointerstitial nephritis and high serum IgM levels. We identified 13 patients with tubulointerstitial nephritis and IgM-positive plasma cell infiltration confirmed with the immunoenzyme method. The clinical findings for these patients included a high prevalence of distal renal tubular acidosis (100%), Fanconi syndrome (92%), and anti-mitochondrial antibodies (82%). The pathologic findings were interstitial nephritis with diffusely distributed CD3-positive T lymphocytes and colocalized IgM-positive plasma cells, as well as tubulitis with CD3-positive T lymphocytes in the proximal tubules and collecting ducts. Additionally, levels of H+-ATPase, H+, K+-ATPase, and the HCO3--Cl- anion exchanger were markedly decreased in the collecting ducts. We propose to designate this group of cases, which have a common histologic and clinical form, as IgM-positive plasma cell-tubulointerstitial nephritis.
Keywords: Fanconi syndrome; IgM; plasma cell; renal tubular acidosis (RTA); tubulointerstitial nephritis (TIN).
Copyright © 2017 by the American Society of Nephrology.
Figures







Similar articles
-
A report of three cases of patients with tubulointerstitial nephritis with IgM-positive plasma cells, treatment, and serum-IgM as a sensitive marker for relapse.BMC Nephrol. 2023 Jul 4;24(1):201. doi: 10.1186/s12882-023-03253-8. BMC Nephrol. 2023. PMID: 37403069 Free PMC article.
-
Tubulointerstitial nephritis with IgM-positive plasma cells complicated by liver failure.CEN Case Rep. 2025 Apr;14(2):253-260. doi: 10.1007/s13730-024-00932-9. Epub 2024 Oct 23. CEN Case Rep. 2025. PMID: 39438434 Free PMC article.
-
Tubulointerstitial nephritis and Fanconi syndrome in a patient with primary Sjögren's syndrome accompanied by antimitochondrial antibodies: A case report and review of the literature.Mod Rheumatol. 2018 Sep;28(5):897-900. doi: 10.3109/14397595.2016.1174422. Epub 2016 May 4. Mod Rheumatol. 2018. PMID: 27142563 Review.
-
Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease.Am J Surg Pathol. 2007 Oct;31(10):1586-97. doi: 10.1097/PAS.0b013e318059b87c. Am J Surg Pathol. 2007. PMID: 17895762
-
Tubulointerstitial nephritis and Fanconi syndrome in primary biliary cirrhosis.Am J Kidney Dis. 2005 Sep;46(3):e41-6. doi: 10.1053/j.ajkd.2005.05.021. Am J Kidney Dis. 2005. PMID: 16129198 Review.
Cited by
-
A patient with chronic kidney disease, primary biliary cirrhosis and metabolic acidosis.Clin Kidney J. 2019 Jun 21;13(3):463-467. doi: 10.1093/ckj/sfz059. eCollection 2020 Jun. Clin Kidney J. 2019. PMID: 32699627 Free PMC article.
-
Primary biliary cholangitis presenting with Fanconi syndrome: an important phenotype.BMJ Case Rep. 2022 Aug 16;15(8):e248461. doi: 10.1136/bcr-2021-248461. BMJ Case Rep. 2022. PMID: 35973749 Free PMC article.
-
Tubulointerstitial Nephritis Cases With IgM-Positive Plasma Cells.Kidney Int Rep. 2020 Jun 18;5(9):1576-1580. doi: 10.1016/j.ekir.2020.06.010. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954083 Free PMC article. No abstract available.
-
IgM-Positive Tubulointerstitial Nephritis Associated With Asymptomatic Primary Biliary Cirrhosis.Kidney Int Rep. 2018 Apr 11;3(4):1004-1009. doi: 10.1016/j.ekir.2018.04.001. eCollection 2018 Jul. Kidney Int Rep. 2018. PMID: 29988993 Free PMC article. No abstract available.
-
Radiation Nephropathy Complicated by Tubulointerstitial Nephritis with Predominantly Lymphocyte and Plasma Cell Infiltration.Intern Med. 2025 Jun 1;64(11):1696-1705. doi: 10.2169/internalmedicine.4265-24. Epub 2024 Nov 1. Intern Med. 2025. PMID: 39496448 Free PMC article.
References
-
- Churg J, Cotran R, Sinniah R, Sakaguchi H, Sobin L: Renal Disease: Classification and Atlas of Tubulo-Interstitial Diseases, Tokyo, Igaku-Shoin, 1985
-
- Colvin R, Fang L: Interstitial nephritis. In: Renal Pathology with Clinical and Functional Correlations, edited by Tisher C, Brenner B, Philadelphia, JB Lippincott, 1994, pp 723–768
-
- Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, Yamamoto M, Takahashi H, Matsui S, Nakada S, Origuchi T, Hirabayashi A, Homma N, Tsubata Y, Takata T, Wada Y, Saito A, Fukase S, Ishioka K, Miyazaki K, Masaki Y, Umehara H, Sugai S, Narita I: Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int 78: 1016–1023, 2010 - PubMed
-
- Saeki T, Kawano M: IgG4-related kidney disease. Kidney Int 85: 251–257, 2014 - PubMed
-
- Takahashi N, Kimura H, Kawajiri Y, Mikami D, Yamamoto C, Kasuno K, Imai N, Kuroda T, Nishi S, Yamamoto M, Yoshida H: Tubulointerstitial nephritis with IgM-positive plasmacytoid large lymphocyte infiltration in a patient with primary biliary cirrhosis and Sjögren’s syndrome. Clin Nephrol 74: 74–80, 2010 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

