Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice
- PMID: 28794154
- PMCID: PMC5633124
- DOI: 10.1074/jbc.M117.790451
Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice
Abstract
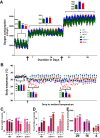
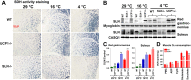
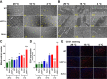
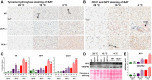
Thermogenesis is an important homeostatic mechanism essential for survival and normal physiological functions in mammals. Both brown adipose tissue (BAT) (i.e. uncoupling protein 1 (UCP1)-based) and skeletal muscle (i.e. sarcolipin (SLN)-based) thermogenesis processes play important roles in temperature homeostasis, but their relative contributions differ from small to large mammals. In this study, we investigated the functional interplay between skeletal muscle- and BAT-based thermogenesis under mild versus severe cold adaptation by employing UCP1-/- and SLN-/- mice. Interestingly, adaptation of SLN-/- mice to mild cold conditions (16 °C) significantly increased UCP1 expression, suggesting increased reliance on BAT-based thermogenesis. This was also evident from structural alterations in BAT morphology, including mitochondrial architecture, increased expression of electron transport chain proteins, and depletion of fat droplets. Similarly, UCP1-/- mice adapted to mild cold up-regulated muscle-based thermogenesis, indicated by increases in muscle succinate dehydrogenase activity, SLN expression, mitochondrial content, and neovascularization, compared with WT mice. These results further confirm that SLN-based thermogenesis is a key player in muscle non-shivering thermogenesis (NST) and can compensate for loss of BAT activity. We also present evidence that the increased reliance on BAT-based NST depends on increased autonomic input, as indicated by abundant levels of tyrosine hydroxylase and neuropeptide Y. Our findings demonstrate that both BAT and muscle-based NST are equally recruited during mild and severe cold adaptation and that loss of heat production from one thermogenic pathway leads to increased recruitment of the other, indicating a functional interplay between these two thermogenic processes.
Keywords: adipose tissue; calcium ATPase; calcium-binding protein; skeletal muscle; uncoupling protein.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures


References
-
- Oelkrug R., Polymeropoulos E. T., and Jastroch M. (2015) Brown adipose tissue: physiological function and evolutionary significance. J. Comp. Physiol. B 185, 587–606 - PubMed
-
- Cannon B., and Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 - PubMed
-
- Nedergaard J., Golozoubova V., Matthias A., Asadi A., Jacobsson A., and Cannon B. (2001) UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta 1504, 82–106 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

