Sequential hemifield gating of α- and β-behavioral performance oscillations after microsaccades
- PMID: 28794193
- PMCID: PMC5680354
- DOI: 10.1152/jn.00253.2017
Sequential hemifield gating of α- and β-behavioral performance oscillations after microsaccades
Abstract
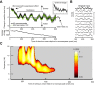
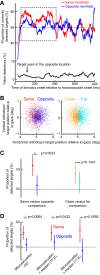
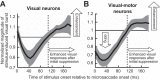
Microsaccades are tiny saccades that occur during gaze fixation. Even though visual processing has been shown to be strongly modulated close to the time of microsaccades, both at central and peripheral eccentricities, it is not clear how these eye movements might influence longer term fluctuations in brain activity and behavior. Here we found that visual processing is significantly affected and, in a rhythmic manner, even several hundreds of milliseconds after a microsaccade. Human visual detection efficiency, as measured by reaction time, exhibited coherent rhythmic oscillations in the α- and β-frequency bands for up to ~650-700 ms after a microsaccade. Surprisingly, the oscillations were sequentially pulsed across visual hemifields relative to microsaccade direction, first occurring in the same hemifield as the movement vector for ~400 ms and then the opposite. Such pulsing also affected perceptual detection performance. Our results suggest that visual processing is subject to long-lasting oscillations that are phase locked to microsaccade generation, and that these oscillations are dependent on microsaccade direction.NEW & NOTEWORTHY We investigated long-term microsaccadic influences on visual processing and found rhythmic oscillations in behavioral performance at α- and β-frequencies (~8-20 Hz). These oscillations were pulsed at a much lower frequency across visual hemifields, first occurring in the same hemifield as the microsaccade direction vector for ~400 ms before switching to the opposite hemifield for a similar interval. Our results suggest that saccades temporally organize visual processing and that such organization can sequentially switch hemifields.
Keywords: fixational eye movements; microsaccades; perceptual oscillations; α-rhythms; β-rhythms.
Copyright © 2017 the American Physiological Society.
Figures








References
-
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for γ-band oscillations. J Neurosci 34: 7639–7644, 2014. doi:10.1523/JNEUROSCI.4216-13.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

