Limbic-motor integration by neural excitations and inhibitions in the nucleus accumbens
- PMID: 28794196
- PMCID: PMC5668465
- DOI: 10.1152/jn.00465.2017
Limbic-motor integration by neural excitations and inhibitions in the nucleus accumbens
Abstract
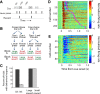
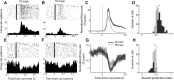
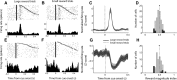
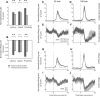
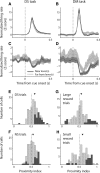
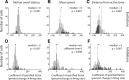
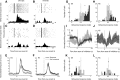
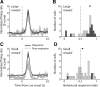
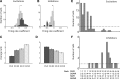
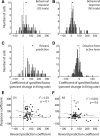
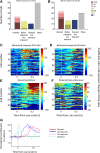
The nucleus accumbens (NAc) has often been described as a "limbic-motor interface," implying that the NAc integrates the value of expected rewards with the motor planning required to obtain them. However, there is little direct evidence that the signaling of individual NAc neurons combines information about predicted reward and behavioral response. We report that cue-evoked neural responses in the NAc form a likely physiological substrate for its limbic-motor integration function. Across task contexts, individual NAc neurons in behaving rats robustly encode the reward-predictive qualities of a cue, as well as the probability of behavioral response to the cue, as coexisting components of the neural signal. In addition, cue-evoked activity encodes spatial and locomotor aspects of the behavioral response, including proximity to a reward-associated target and the latency and speed of approach to the target. Notably, there are important limits to the ability of NAc neurons to integrate motivational information into behavior: in particular, updating of predicted reward value appears to occur on a relatively long timescale, since NAc neurons fail to discriminate between cues with reward associations that change frequently. Overall, these findings suggest that NAc cue-evoked signals, including inhibition of firing (as noted here for the first time), provide a mechanism for linking reward prediction and other motivationally relevant factors, such as spatial proximity, to the probability and vigor of a reward-seeking behavioral response.NEW & NOTEWORTHY The nucleus accumbens (NAc) is thought to link expected rewards and action planning, but evidence for this idea remains sparse. We show that, across contexts, both excitatory and inhibitory cue-evoked activity in the NAc jointly encode reward prediction and probability of behavioral responding to the cue, as well as spatial and locomotor properties of the response. Interestingly, although spatial information in the NAc is updated quickly, fine-grained updating of reward value occurs over a longer timescale.
Keywords: electrophysiology; locomotion; motivation; nucleus accumbens; reward.
Copyright © 2017 the American Physiological Society.
Figures

Similar articles
-
Nucleus accumbens dopamine encodes the trace period during appetitive Pavlovian conditioning.bioRxiv [Preprint]. 2025 Apr 3:2025.01.07.631806. doi: 10.1101/2025.01.07.631806. bioRxiv. 2025. Update in: eNeuro. 2025 May 27;12(5):ENEURO.0016-25.2025. doi: 10.1523/ENEURO.0016-25.2025. PMID: 40235964 Free PMC article. Updated. Preprint.
-
Modulation of Dopamine Neurons Alters Behavior and Event Encoding in the Nucleus Accumbens during Pavlovian Conditioning.J Neurosci. 2025 Jun 25;45(26):e0061252025. doi: 10.1523/JNEUROSCI.0061-25.2025. J Neurosci. 2025. PMID: 40393804
-
Paraventricular Nucleus of the Thalamus Neurons That Project to the Nucleus Accumbens Show Enhanced c-Fos Expression During Early-Stage Cue-Reward Associative Learning in Male Rats.Eur J Neurosci. 2025 Jun;61(12):e70168. doi: 10.1111/ejn.70168. Eur J Neurosci. 2025. PMID: 40542605 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Incentives for preventing smoking in children and adolescents.Cochrane Database Syst Rev. 2017 Jun 6;6(6):CD008645. doi: 10.1002/14651858.CD008645.pub3. Cochrane Database Syst Rev. 2017. PMID: 28585288 Free PMC article.
Cited by
-
Where Actions Meet Outcomes: Medial Prefrontal Cortex, Central Thalamus, and the Basal Ganglia.Front Behav Neurosci. 2022 Jul 5;16:928610. doi: 10.3389/fnbeh.2022.928610. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35864847 Free PMC article. Review.
-
Sign Tracking and Goal Tracking Are Characterized by Distinct Patterns of Nucleus Accumbens Activity.eNeuro. 2019 Mar 15;6(2):ENEURO.0414-18.2019. doi: 10.1523/ENEURO.0414-18.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 30886890 Free PMC article.
-
Effects of Immediate Aversive Stimulation on Haloperidol-Induced Catalepsy in Rats.Front Behav Neurosci. 2022 Apr 11;16:867180. doi: 10.3389/fnbeh.2022.867180. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35481243 Free PMC article.
-
Nucleus Accumbens Shell Neurons Encode the Kinematics of Reward Approach Locomotion.Neuroscience. 2023 Aug 1;524:181-196. doi: 10.1016/j.neuroscience.2023.06.002. Epub 2023 Jun 15. Neuroscience. 2023. PMID: 37330195 Free PMC article.
-
The Nucleus Accumbens Core Is Necessary for Responding to Incentive But Not Instructive Stimuli.J Neurosci. 2020 Feb 5;40(6):1332-1343. doi: 10.1523/JNEUROSCI.0194-19.2019. Epub 2019 Dec 20. J Neurosci. 2020. PMID: 31862857 Free PMC article.
References
-
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol B 34: 77–98, 1982. doi: 10.1080/14640748208400878. - DOI
-
- Adams CD, Dickinson A. Instrumental responding following reinforcer devaluation. Q J Exp Psychol B 33: 109–121, 1981. doi: 10.1080/14640748108400816. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

