Reconstituted IMPDH polymers accommodate both catalytically active and inactive conformations
- PMID: 28794265
- PMCID: PMC5620369
- DOI: 10.1091/mbc.E17-04-0263
Reconstituted IMPDH polymers accommodate both catalytically active and inactive conformations
Abstract
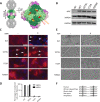
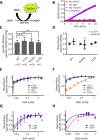
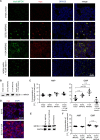
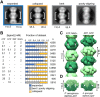
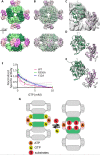
Several metabolic enzymes undergo reversible polymerization into macromolecular assemblies. The function of these assemblies is often unclear but in some cases they regulate enzyme activity and metabolic homeostasis. The guanine nucleotide biosynthetic enzyme inosine monophosphate dehydrogenase (IMPDH) forms octamers that polymerize into helical chains. In mammalian cells, IMPDH filaments can associate into micron-length assemblies. Polymerization and enzyme activity are regulated in part by binding of purine nucleotides to an allosteric regulatory domain. ATP promotes octamer polymerization, whereas GTP promotes a compact, inactive conformation whose ability to polymerize is unknown. Also unclear is whether polymerization directly alters IMPDH catalytic activity. To address this, we identified point mutants of human IMPDH2 that either prevent or promote polymerization. Unexpectedly, we found that polymerized and non-assembled forms of recombinant IMPDH have comparable catalytic activity, substrate affinity, and GTP sensitivity and validated this finding in cells. Electron microscopy revealed that substrates and allosteric nucleotides shift the equilibrium between active and inactive conformations in both the octamer and the filament. Unlike other metabolic filaments, which selectively stabilize active or inactive conformations, recombinant IMPDH filaments accommodate multiple states. These conformational states are finely tuned by substrate availability and purine balance, while polymerization may allow cooperative transitions between states.
© 2017 by The American Society for Cell Biology.
Figures
References
-
- Beaty NB, Lane MD. The polymerization of acetyl-CoA carboxylase. J Biol Chem. 1983;258:13051–13055. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

