Living GenoChemetics by hyphenating synthetic biology and synthetic chemistry in vivo
- PMID: 28794415
- PMCID: PMC5550429
- DOI: 10.1038/s41467-017-00194-3
Living GenoChemetics by hyphenating synthetic biology and synthetic chemistry in vivo
Abstract
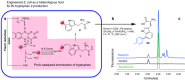
Marrying synthetic biology with synthetic chemistry provides a powerful approach toward natural product diversification, combining the best of both worlds: expediency and synthetic capability of biogenic pathways and chemical diversity enabled by organic synthesis. Biosynthetic pathway engineering can be employed to insert a chemically orthogonal tag into a complex natural scaffold affording the possibility of site-selective modification without employing protecting group strategies. Here we show that, by installing a sufficiently reactive handle (e.g., a C-Br bond) and developing compatible mild aqueous chemistries, synchronous biosynthesis of the tagged metabolite and its subsequent chemical modification in living culture can be achieved. This approach can potentially enable many new applications: for example, assay of directed evolution of enzymes catalyzing halo-metabolite biosynthesis in living cells or generating and following the fate of tagged metabolites and biomolecules in living systems. We report synthetic biological access to new-to-nature bromo-metabolites and the concomitant biorthogonal cross-coupling of halo-metabolites in living cultures.Coupling synthetic biology and chemical reactions in cells is a challenging task. The authors engineer bacteria capable of generating bromo-metabolites, develop a mild Suzuki-Miyaura cross-coupling reaction compatible with cell growth and carry out the cross-coupling chemistry in live cell cultures.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
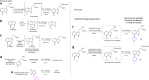
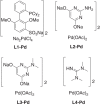
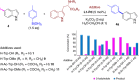
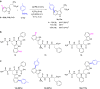

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

