Inhibition of Endoplasmic Reticulum Stress Preserves the Integrity of Blood-Spinal Cord Barrier in Diabetic Rats Subjected to Spinal Cord Injury
- PMID: 28794417
- PMCID: PMC5550423
- DOI: 10.1038/s41598-017-08052-4
Inhibition of Endoplasmic Reticulum Stress Preserves the Integrity of Blood-Spinal Cord Barrier in Diabetic Rats Subjected to Spinal Cord Injury
Erratum in
-
Author Correction: Inhibition of Endoplasmic Reticulum Stress Preserves the Integrity of Blood-Spinal Cord Barrier in Diabetic Rats Subjected to Spinal Cord Injury.Sci Rep. 2022 Feb 10;12(1):2629. doi: 10.1038/s41598-022-06845-w. Sci Rep. 2022. PMID: 35145220 Free PMC article. No abstract available.
Abstract
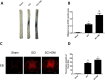
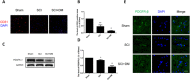
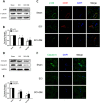
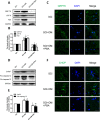
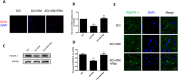
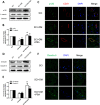
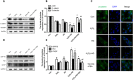
The blood-spinal cord barrier (BSCB) plays significance roles in recovery following spinal cord injury (SCI), and diabetes mellitus (DM) impairs endothelial cell function and integrity of BSCS. Endoplasmic reticulum (ER) stress occurs in the early stages of SCI and affects prognosis and cell survival. However, the relationship between ER stress and the integrity of BSCB in diabetic rats after SCI remains unclear. Here we observed that diabetic rats showed increased extravasation of Evans Blue (EB) dye, and loss of endothelial cells and pericytes 1 day after SCI compared to non-diabetic rats. Diabetes was also shown to induce activation of ER stress. Similar effects were observed in human brain microvascular endothelial cells. 4-phenylbutyric acid (4-PBA), an ER stress inhibitor lowered the adverse effect of diabetes on SCI, reduced EB dye extravasation, and limited the loss of endothelial cells and pericytes. Moreover, 4-PBA treatment partially reversed the degradation of tight junction and adherens junction both in vivo and in vitro. In conclusion, diabetes exacerbates the disruption of BSCB after SCI via inducing ER stress, and inhibition of ER stress by 4-PBA may play a beneficial role on the integrity of BSCB in diabetic SCI rats, leading to improved prognosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Mikami Y, et al. A simple and reliable behavioral analysis of locomotor function after spinal cord injury in mice. Technical note. Journal of neurosurgery. 2002;97:142–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

