Tumor infiltrating leukocyte density is independent of tumor grade and molecular subtype in aggressive breast cancer of Western Kenya
- PMID: 28794686
- PMCID: PMC5543450
- DOI: 10.1186/s41182-017-0059-4
Tumor infiltrating leukocyte density is independent of tumor grade and molecular subtype in aggressive breast cancer of Western Kenya
Abstract
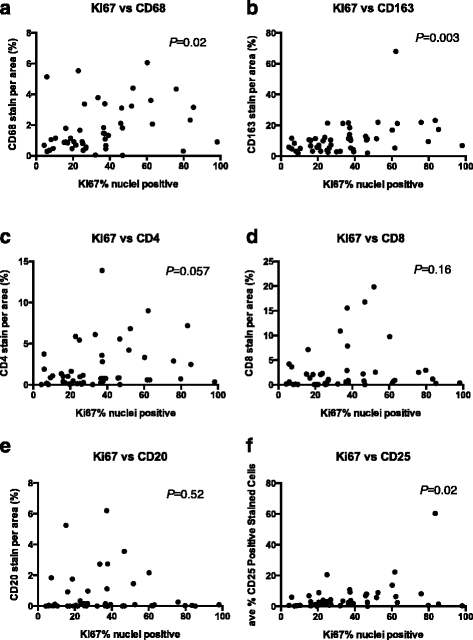
Background: Tumors commonly are infiltrated by leukocytes, or tumor infiltrating leukocytes (TILs). It remains unclear, however, if the density and type of individual TILs has a direct or simply correlative role in promoting poor prognosis in breast cancer patients. Breast cancer in Kenyan women is aggressive with presentation at a young age, with advanced grade (grade III), large tumor size (>2.0 cm), and poor prognosis. We previously observed that the tumors were predominantly estrogen receptor positive (ER+) but also included both a high percentage of triple negative tumors and also increased immune cell infiltration within the tumors. We used breast tumor tissues from each patient to make tissue microarrays that were then stained for leukocyte and myeloid markers including CD4, CD8, CD20, CD25, CD68, and CD163 using immunohistochemical techniques. The immune cell infiltration into the cancer tissue included increased numbers of macrophages (CD68+), helper T cells (CD4+), and CD25+ lymphocytes compared to benign tissue.
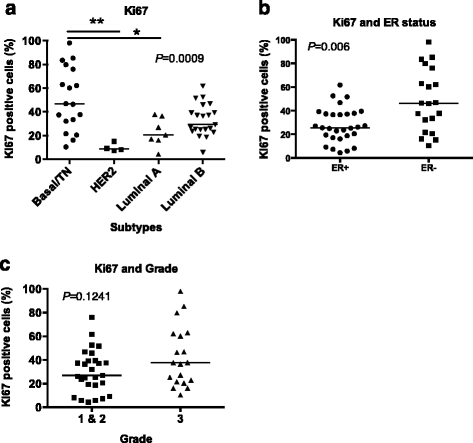
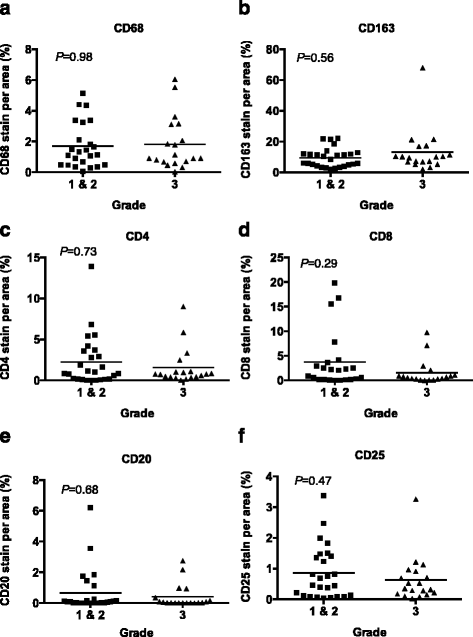
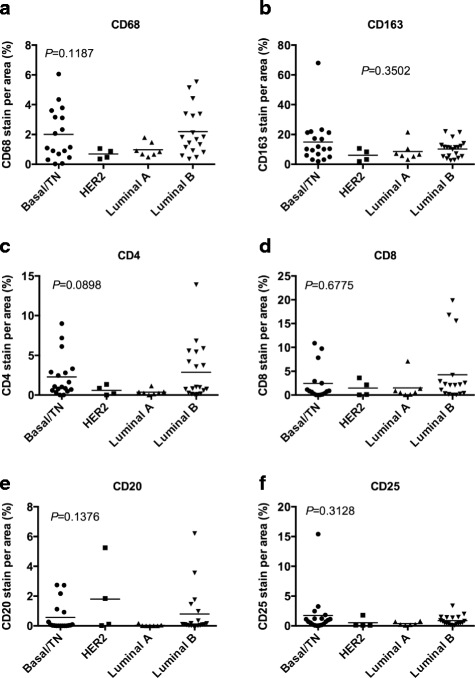
Results: This study characterized the grade, molecular subtypes, and proliferation index of these tumors and determined if TIL density was enriched across any of these factors. We analyzed 49 malignant patient tissue samples for this study. The patient population had a mean age of 51.9 years. The tumors analyzed were heterogeneous by grade: grade I (6%), grade II (47%), and grade III (39%). Most patients presented with large tumors (>2.0 cm) (69%). We classified the tumors into molecular subtypes based on clinical marker expression. Based on this analysis, the molecular subtype distribution was heterogeneous with luminal B (41%), basal/triple negative (TN) (37%), luminal A (14%) and HER2 (8%) breast cancer subtypes. While the basal/TN subtype had a much higher proliferative index (Ki-67+) than did the other molecular subtypes, we did not see a significant correlation between TIL density and either subtype or tumor grade. Therefore, TIL density is independent of molecular subtype and grade.
Conclusion: This study identified a Kenyan patient cohort that develops large, high-grade tumors primarily of the luminal B and basal molecular subtypes. After analyzing the TILs within these tumors, we found that immune cell infiltration of these tumors correlated with increased proliferation but not grade or molecular subtype. Future research is required to determine if the aberrant recruitment of TILs to tumors contributes to cancer progression and response to cancer treatments.
Keywords: Advanced; African; Aggressive breast cancer; Breast cancer subtypes; Kenyan; Tumor infiltrating leukocytes (TILs).
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Institutional Ethics and Review Committee of Moi Teaching and Referral Hospital (MTRH) and the University of Notre Dame Institutional Review Board and was conducted according to the principles in the Declaration of Helsinki (Deutsch 1989).
Patients with histologically diagnosed breast cancer at the MTRH were included in the study after giving their informed consent and have been described previously [12].
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, De Azambuja E. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–7. doi: 10.1200/JCO.2011.41.0902. - DOI - PubMed
-
- Denkert C, Huober J, Loibl S, Prinzler J, Kronenwett R, Darb-Esfahani S, Brase JC, Solbach C, Mehta K, Fasching PA, Sinn BV. HER2 and ESR1 mRNA expression levels and response to neoadjuvant trastuzumab plus chemotherapy in patients with primary breast cancer. Breast Cancer Res. 2013;15(1):R11. doi: 10.1186/bcr3384. - DOI - PMC - PubMed
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. - DOI - PubMed
-
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
