WITHDRAWN: Intermittent catheterisation for long-term bladder management
- PMID: 28796279
- PMCID: PMC6483323
- DOI: 10.1002/14651858.CD006008.pub4
WITHDRAWN: Intermittent catheterisation for long-term bladder management
Update in
-
Intermittent catheter techniques, strategies and designs for managing long-term bladder conditions.Cochrane Database Syst Rev. 2021 Oct 26;10(10):CD006008. doi: 10.1002/14651858.CD006008.pub5. Cochrane Database Syst Rev. 2021. PMID: 34699062 Free PMC article.
Abstract
Background: Intermittent catheterisation is a commonly recommended procedure for people with incomplete bladder emptying. There are now several designs of intermittent catheter (e.g. different lengths, 'ready to use' presentation) with different materials (e.g. PVC-free) and coatings (e.g. hydrophilic). The most frequent complication of intermittent catheterisation is urinary tract infection (UTI), but satisfaction, preference and ease of use are also important to users. It is unclear which catheter designs, techniques or strategies affect the incidence of UTI, which are preferable to users and which are most cost effective.
Objectives: To compare one type of catheter design versus another, one type of catheter material versus another, aseptic catheterisation technique versus clean technique, single-use (sterile) catheters versus multiple-use (clean) catheters, self-catheterisation versus catheterisation by others and any other strategies designed to reduce UTI and other complications or improve user-reported outcomes (user satisfaction, preference, ease of use) and cost effectiveness in adults and children using intermittent catheterisation for incomplete bladder emptying.
Search methods: We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, and handsearching of journals and conference proceedings (searched 30 September 2013), the reference lists of relevant articles and conference proceedings, and we attempted to contact other investigators for unpublished data or for clarification.
Selection criteria: Randomised controlled trials (RCTs) or randomised cross-over trials comparing at least two different catheter designs, catheterisation techniques or strategies.
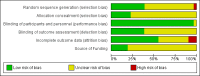
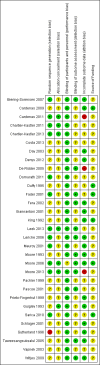
Data collection and analysis: Two review authors assessed the methodological quality of trials and abstracted data. For dichotomous variables, risk ratios and 95% confidence intervals were derived for each outcome where possible. For continuous variables, mean differences and 95% confidence intervals were calculated for each outcome. Because of trial heterogeneity, it was not always possible to combine data to give an overall estimate of treatment effect.
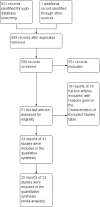
Main results: Thirty-one trials met the inclusion criteria, including 13 RCTs and 18 cross-over trials. Most were small (less than 60 participants completed), although five trials had more than 100 participants. There was considerable variation in length of follow-up and definitions of UTI. Participant dropout was a problem for several trials, particularly where there was long-term follow-up to measure incidence of UTI. Fifteen trials were more than 10 years old and focused mainly on comparing different catheterisation techniques (e.g. single versus multiple-use) on clinical outcomes whereas, several more recent trials have focused on comparing different types of catheter designs or materials, especially coatings, and user preference. It was not possible to combine data from some trials owing to variations in the catheters tested and in particular the catheter coatings. Where there were data, confidence intervals around estimates were wide and hence clinically important differences in UTI and other outcomes could neither be identified nor reliably ruled out. No study assessed cost-effectiveness.
Authors' conclusions: Despite a total of 31 trials, there is still no convincing evidence that the incidence of UTI is affected by use of aseptic or clean technique, coated or uncoated catheters, single (sterile) or multiple-use (clean) catheters, self-catheterisation or catheterisation by others, or by any other strategy. Results from user-reported outcomes varied. The current research evidence is weak and design issues are significant. More well-designed trials are strongly recommended. Such trials should include analysis of cost-effectiveness because there are likely to be substantial differences associated with the use of different catheter designs, catheterisation techniques and strategies.
Conflict of interest statement
Katherine Moore was a co‐investigator on a trial sponsored by Coloplast (Cardenas 2011) and received product from Coloplast for another trial (Moore 2013). Mandy Fader has received intermittent catheter products for research purposes from Astra Tech AB.
Figures





































Update of
-
Intermittent catheterisation for long-term bladder management.Cochrane Database Syst Rev. 2014 Sep 10;(9):CD006008. doi: 10.1002/14651858.CD006008.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Aug 08;8:CD006008. doi: 10.1002/14651858.CD006008.pub4. PMID: 25208303 Updated.
References
References to studies included in this review
-
- Biering‐Sorensen F, Hansen HV, Nielsen PN, Looms D. Residual urine after intermittent catheterization in females using two different catheters. Scandinavian Journal of Urology and Nephrology 2007;41:341‐5. - PubMed
-
- Cardenas DD, Hoffman JM. Hydrophilic catheters versus noncoated catheters for reducing the Incidence of urinary tract infections: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2009;90(10):1668‐71. - PubMed
-
- Cardenas DD, Moore KN, Dannels‐McClure A, Scelza WM, Graves DE, Brooks M, et al. Intermittent catheterization with a hydrophilic‐coated catheter delays urinary tract infections in acute spinal injury: a prospective randomised, multicenter trial. Physical Medicine and Rehabilitation 2011;3:408‐17. - PubMed
-
- Chartier‐Kastler E, Lauge I, Ruffion A, Goossens D, Charvier K, Biering‐Sorensen F. Safety of a new compact catheter for men with neurogenic bladder dysfunction: a randomised, crossover and open‐labelled study. Spinal Cord 2011;49(7):844‐50. - PubMed
-
- Chartier‐Kastler E, Amarenco G, Lindbo L, Soljanik I, Andersen HL, Bagi P, et al. A prospective, randomized, crossover, multicenter study comparing quality of life using compact versus standard catheters for intermittent self‐catheterization. Journal of Urology 2013;190(3):942‐7. - PubMed
References to studies excluded from this review
-
- Bagi P, Hannibalsen J, Permild R, Stilling S, Looms D. Safety of a new compact male intermittent catheter: randomized, cross‐over, single‐blind study in healthy male volunteers. Urology International 2011;86(2):179‐84. - PubMed
-
- Bennett C, Young M, Razi S, Adkins R, Diaz F, McCrary A. The effect of urethral introducer tip catheters on the incidence of urinarytract infection outcomes in spinal cord injured patients. Journal of Urology 1997;158(2):519‐21. - PubMed
-
- Charbonneau‐Smith R. No‐touch catheterization and infection rates in a select spinal cord injured population. Rehabilitation Nursing 1993;18:296‐9,305,355‐6. [2282] - PubMed
-
- Clarke SA, Samuel M, Boddy S. Are prophylactic antibiotics necessary with clean intermittent catheterization? A randomised controlled trial. Journal of Pediatric Surgery 2005;40(3):568‐71. - PubMed
-
- Diokno AC, Mitchell BA, Nash AJ, Kimbrough JA. Patient satisfaction and the LoFric catheter for clean intermittent catheterization. Journal of Urology 1995;153(2):349‐51. - PubMed
Additional references
-
- Campbell J, Moore KN, Voaklander D, Mix, L. Complications associated with clean intermittent catheterization in children with spina bifida. Journal of Urology 2004;171(6 Pt 1):2420‐2. - PubMed
-
- Cottenden A, Bliss DZ, Buckley B, Fader M, Gartley C, Hayder D, et al. Management using continence products. In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence: 5th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and Treatment of Urinary Incontinence, Pelvic Organ Prolapse and Faecal Incontinence; 2012 Feb 23‐25; Paris. 5th Edition. Belgium: International Consultation on Urological Diseases (ICUD), 2013:1651‐786.
-
- Li L, Ye W, Ruan H, Yang B, Zhang S, Li L. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta‐analysis of randomized controlled trials. Archives of Physical Medicine and Rehabilitation 2013;94(4):782‐7. - PubMed
-
- National Institute on Disability and Rehabilitation Research (NIDRR). The prevention and management of urinary tract infections among people with spinal cord injuries. National Institute on Disability and Rehabilitation Research Consensus Statement. January 27‐29, 1992. Journal of the American Paraplegia Society 1992;15(3):194‐204. - PubMed
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

