Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy
- PMID: 28796684
- PMCID: PMC6496947
- DOI: 10.1097/AOG.0000000000002191
Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy
Abstract
Objective: To evaluate whether quadrivalent human papillomavirus vaccine (4vHPV) administered during the periconceptional period or during pregnancy was associated with increased risks for adverse obstetric events, adverse birth outcomes, or selected major structural birth defects.
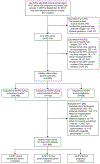
Methods: We conducted a retrospective, observational cohort study using administrative and health care data from the Vaccine Safety Datalink. Insured women 13-27 years old with singleton pregnancies and a live birth from January 1, 2007, through September 1, 2013, who received 4vHPV during the periconceptional period (2 weeks before to 2 weeks after their last menstrual period), during pregnancy, or during both periods combined were compared with women who had a live birth during the same time period and received 4vHPV 4-18 months before their last menstrual period. We examined risks of gestational diabetes, hypertensive disorders of pregnancy, chorioamnionitis, preterm birth, small-for-gestational-age birth, and selected major structural birth defects in offspring. We estimated relative risks associated with receipt of 4vHPV during the periconceptional period, during pregnancy, and both exposure periods combined using a generalized linear model with Poisson distribution including a propensity score that included relevant maternal demographic and pregnancy characteristics.
Results: Of 92,579 potentially eligible pregnant women, 720 received 4vHPV during the periconceptional period, 638 received 4vHPV during pregnancy, and 8,196 received 4vHPV during the comparison period. Administration of 4vHPV during pregnancy was not associated with increased risk of adverse obstetric events, birth outcomes. Preterm birth occurred in 7.9% of pregnancies with vaccine exposures during pregnancy compared with 7.6% of pregnancies with vaccination in the comparison period (adjusted relative risk 0.97, 95% CI 0.72-1.3). Major structural birth defects were diagnosed in 2.0% of pregnancies with vaccine exposure during pregnancy compared with 1.8% of pregnancies with vaccine exposure during the comparison period (adjusted prevalence ratio 1.0, 95% CI 0.52-1.9). Results were similar for 4vHPV exposure during the periconceptional period.
Conclusion: Quadrivalent HPV vaccine inadvertently administered in pregnancy or during the periconceptional period was not associated with adverse pregnancy or birth outcomes.
Figures
References
-
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187–93. - PubMed
-
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998;338:423–8. - PubMed
-
- Smith LM, Strumpf EC, Kaufman JS, Lofters A, Schwandt M, Levesque LE. The early benefits of human papillomavirus vaccination on cervical dysplasia and anogenital warts. Pediatrics 2015;135:e1131–40. - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

