Histological study on maturation, fertilization and the state of gonadal region following spawning in the model sea anemone, Nematostella vectensis
- PMID: 28796817
- PMCID: PMC5552035
- DOI: 10.1371/journal.pone.0182677
Histological study on maturation, fertilization and the state of gonadal region following spawning in the model sea anemone, Nematostella vectensis
Abstract
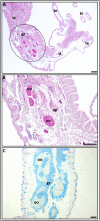
The starlet sea-anemone Nematostella vectensis has emerged as a model organism in developmental biology. Still, our understanding of various biological features, including reproductive biology of this model species are in its infancy. Consequently, through histological sections, we study here key stages of the oogenesis (oocyte maturation/fertilization), as the state of the gonad region immediately after natural spawning. Germ cells develop in a secluded mesenterial gastrodermal zone, where the developing oocytes are surrounded by mucoid glandular cells and trophocytes (accessory cells). During vitellogenesis, the germinal vesicle in oocytes migrates towards the animal pole and the large polarized oocytes begin to mature, characterized by karyosphere formation. Then, the karyosphere breaks down, the chromosomes form the metaphase plate I and the eggs are extruded from the animal enclosed in a sticky, jelly-like mucoid mass, along with numerous nematosomes. Fertilization occurs externally at metaphase II via swimming sperm extruded by males during natural spawning. The polar bodies are ejected from the eggs and are situated within a narrow space between the egg's vitelline membrane and the adjacent edge of the jelly coat. The cortical reaction occurs only at the polar bodies' ejection site. Several spermatozoa can penetrate the same egg. Fertilization is accompanied by a strong ooplasmatic segregation. Immediately after spawning, the gonad region holds many previtellogenic and vitellogenic oocytes, though no oocytes with karyosphere. Above are the first histological descriptions for egg maturation, meiotic chromosome's status at fertilization, fertilization and the gonadal region's state following spawning, also documenting for the first time the ejection of the polar body.
Conflict of interest statement
Figures





References
-
- Williams RB. A redescription of the brackish water sea-anemone Nematostella vectensis Stephenson, with an appraisal of co-generic species. J Nat Hist 1975; 9: 51–64.
-
- Hand C, Uhlinger KR. The unique, widely distributed estuarine sea anemone, Nematostella vectensis Stephenson: a review new facts, and questions. Estuaries 1994; 17: 501–508.
-
- Daly M. A systematic revision of Edvardsiidae (Cnidaria, Anthozoa). Invert Biol 2002; 121: 212–225.
-
- Sullivan JC, Ryan JF, Watson JA, Webb J, Mullikin JC, Rokhsar D, et al. Stellabase: the Nematostella vectensis genomics database. Nucleic Acids Res 2006; 34: D495–499. doi: 10.1093/nar/gkj020 - DOI - PMC - PubMed
-
- Eckelbarger KJ, Hand C, Uhlinger KR. Ultrastructural features of the trophonema and oogenesis in the starlet sea anemone, Nematostella vectensis (Edvardsiidae). Invert Biol 2008; 127: 381–395.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

