Telephone-Based Depression Care Management for Postpartum Women: A Randomized Controlled Trial
- PMID: 28796940
- PMCID: PMC7295181
- DOI: 10.4088/JCP.15m10563
Telephone-Based Depression Care Management for Postpartum Women: A Randomized Controlled Trial
Abstract
Objective: With a period prevalence of 21.9% in the year after birth, depression is a common complication of childbearing. We assessed the impact of telephone-delivered depression care management (DCM) on symptom levels, health service utilization, and functional status 3, 6, and 12 months postpartum.
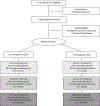
Methods: The randomized controlled trial was conducted at the University of Pittsburgh, Pittsburgh, Pennsylvania, from March 2006 through September 2010. Women (N = 628) who screened positive for depression (a score of 10 or greater on the Edinburgh Postnatal Depression Scale) 4 to 6 weeks postpartum were evaluated with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen and enrolled in a randomized trial of DCM compared to enhanced usual care (EUC). Clinicians conducted telephone contacts to educate, assist with treatment decisions, monitor symptoms, facilitate access to services, and encourage links to community resources. Independent evaluators collected symptom scores, functional status, and health services use at 3, 6, and 12 months postpartum. Primary outcome was reduction of symptoms as measured by the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement.
Results: Mean depressive symptom and function scores significantly improved (by greater than 50%) in both groups of women but did not differ by DCM versus EUC assignment. Health services use was similar in women randomly assigned to DCM compared to EUC. Women with childhood sexual abuse responded significantly more favorably to DCM on depression and functional measures (all P values < .02).
Conclusions: Both DCM and EUC favorably impacted depression symptom levels and function. The subgroup of women with childhood sexual abuse benefited significantly more from DCM compared to the EUC condition. Regular telephone availability of a clinician is a resource that appears to be particularly therapeutic to women with childhood sexual abuse.
Trial registration: ClinicalTrials.gov identifier: NCT00282776.
© Copyright 2017 Physicians Postgraduate Press, Inc.
Conflict of interest statement
Figures
References
-
- Miller LJ. Postpartum depression. JAMA. 2002;287(6):762–765. - PubMed
-
- Wisner KL, Parry BL, Piontek CM. Clinical practice: postpartum depression. N Engl J Med. 2002;347(3):194–199. - PubMed
-
- Milgrom J, Gemmill AW. Screening for perinatal depression. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):13–23. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

