Uterotubal junction prevents chlamydial ascension via innate immunity
- PMID: 28797102
- PMCID: PMC5552320
- DOI: 10.1371/journal.pone.0183189
Uterotubal junction prevents chlamydial ascension via innate immunity
Abstract
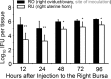
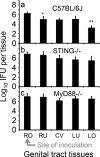
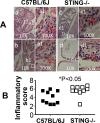
Ascension to the oviduct is necessary for Chlamydia to induce tubal infertility. Using the Chlamydia muridarum induction of hydrosalpinx mouse model, we have demonstrated a significant role of the uterotubal junction in preventing chlamydial ascending infection. First, delivery of C. muridarum to either side of the uterotubal junction resulted in significant reduction in live organisms from the tissues on the opposite sides. However, the recovery yields remained similar among different sections of the uterine horn. These observations suggest that the uterotubal junction may function as a barrier between the uterine horn and oviduct. Second, deficiency in innate immunity signaling pathways mediated by either MyD88 or STING significantly compromised the uterotubal junction barrier function, permitting C. muridarum to spread freely between uterine horn and oviduct. Finally, transcervical inoculation of C. muridarum led to significantly higher incidence of bilateral hydrosalpinges in the STING-deficient mice while the same inoculation mainly induced unilateral hydrosalpinx in the wild type mice, suggesting that the STING pathway-dependent uterotubal junction plays a significant role in preventing tubal pathology. Thus, we have demonstrated for the first time that the uterotubal junction is a functional barrier for preventing tubal infection by a sexually transmitted agent, providing the first in vivo evidence for detecting chlamydial infection by the STING pathway.
Conflict of interest statement
Figures





Similar articles
-
Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis.Trends Microbiol. 2018 Jul;26(7):611-623. doi: 10.1016/j.tim.2017.12.002. Epub 2017 Dec 27. Trends Microbiol. 2018. PMID: 29289422 Free PMC article. Review.
-
Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum.PLoS One. 2013 Aug 5;8(8):e71649. doi: 10.1371/journal.pone.0071649. Print 2013. PLoS One. 2013. PMID: 23940777 Free PMC article.
-
The p47phox deficiency significantly attenuates the pathogenicity of Chlamydia muridarum in the mouse oviduct but not uterine tissues.Microbes Infect. 2016 Mar;18(3):190-8. doi: 10.1016/j.micinf.2015.11.003. Epub 2015 Dec 2. Microbes Infect. 2016. PMID: 26645958
-
Chlamydia muridarum plasmid induces mouse oviduct pathology by promoting chlamydial survival and ascending infection and triggering host inflammation.Eur J Dermatol. 2018 Oct 1;28(5):628-636. doi: 10.1684/ejd.2018.3399. Eur J Dermatol. 2018. PMID: 30442635
-
Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections.Contraception. 2015 Aug;92(2):108-15. doi: 10.1016/j.contraception.2015.01.004. Epub 2015 Jan 13. Contraception. 2015. PMID: 25592078 Review.
Cited by
-
A Nonsurgical Embryo Transfer Technique for Fresh and Cultured Blastocysts in Rats.J Am Assoc Lab Anim Sci. 2020 Sep 1;59(5):488-495. doi: 10.30802/AALAS-JAALAS-19-000163. Epub 2020 Aug 12. J Am Assoc Lab Anim Sci. 2020. PMID: 32787997 Free PMC article.
-
Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis.Trends Microbiol. 2018 Jul;26(7):611-623. doi: 10.1016/j.tim.2017.12.002. Epub 2017 Dec 27. Trends Microbiol. 2018. PMID: 29289422 Free PMC article. Review.
-
Immunopathogenesis of genital Chlamydia infection: insights from mouse models.Pathog Dis. 2021 Mar 31;79(4):ftab012. doi: 10.1093/femspd/ftab012. Pathog Dis. 2021. PMID: 33538819 Free PMC article. Review.
-
Evidence for cGAS-STING Signaling in the Female Genital Tract Resistance to Chlamydia trachomatis Infection.Infect Immun. 2022 Feb 17;90(2):e0067021. doi: 10.1128/iai.00670-21. Epub 2022 Jan 3. Infect Immun. 2022. PMID: 34978925 Free PMC article.
References
-
- Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, et al. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstetrics and gynecology. 2012;119(5):1009–16. doi: 10.1097/AOG.0b013e3182519326 ; PubMed Central PMCID: PMC4608258. - DOI - PMC - PubMed
-
- Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, et al. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril. 2011;96(3):715–21. Epub 2011/07/12. S0015-0282(11)00966-6 [pii] doi: 10.1016/j.fertnstert.2011.06.021 . - DOI - PMC - PubMed
-
- Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, et al. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P. American journal of obstetrics and gynecology. 2010;203(5):494 e7– e14. Epub 2010/07/21. S0002-9378(10)00705-2 [pii] doi: 10.1016/j.ajog.2010.06.005 . - DOI - PMC - PubMed
-
- Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, et al. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis. 2005;32(1):49–56. Epub 2004/12/23. 00007435-200501000-00008 [pii]. . - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

