Survival benefit of transarterial chemoembolization in patients with metastatic hepatocellular carcinoma: a single center experience
- PMID: 28797231
- PMCID: PMC5553671
- DOI: 10.1186/s12876-017-0656-z
Survival benefit of transarterial chemoembolization in patients with metastatic hepatocellular carcinoma: a single center experience
Abstract
Background: As prognosis of patients with metastatic hepatocellular carcinoma (HCC) is mainly determined by intrahepatic HCC progression, local treatment with TACE may result in improved OS, although it is not recommended. The purpose of this study was to analyze retrospectively the efficacy of TACE and its impact on OS in patients with metastatic hepatocellular carcinoma (HCC).
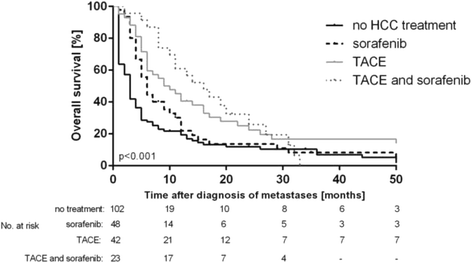
Methods: Two hundred and fifteen patients with metastatic HCC who were treated at our Liver Center between 2003 and 2014 were included in this retrospective analysis. Medical records, laboratory parameters and imaging studies were analyzed. Treatment of metastatic HCC and OS were assessed RESULTS: One hundred and two patients (47.4%) did not receive any HCC specific treatment while 48 patients (22.3%) were treated with sorafenib, 42 patients (19.5%) with TACE and 23 patients (10.7%) received treatment with TACE and sorafenib in combination. Survival analyses and Cox regression models revealed that TACE and a combination therapy of TACE and sorafenib were significant prognostic factors in metastatic HCC. However, further analyses revealed that there was no additional prognostic effect of adding sorafenib to TACE treatment in this patient cohort.
Conclusions: In metastatic HCC, treatment of intrahepatic tumor by TACE may be associated with improved survival. These results support the prognostic importance of treating intrahepatic HCC even in patients with metastatic disease. Therefore, we suggest evaluating the technical feasibility of TACE in all metastatic patients.
Keywords: Hepatocellular carcinoma; Metastases; Prognosis; Sorafenib; Transarterial chemoembolization.
Conflict of interest statement
Ethics approval and consent to participate
All patients provided written informed consent for TACE and for data collection. This study was performed in accordance with the Declaration of Helsinki and it has been approved by the local ethics committee of the University Hospital of Freiburg (no. EK 62/14).
Consent for publication
Not applicable.
Competing interests
DB receives teaching and speaking fees from Bayer HealthCare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

