Using smartphones to decrease substance use via self-monitoring and recovery support: study protocol for a randomized control trial
- PMID: 28797307
- PMCID: PMC5553728
- DOI: 10.1186/s13063-017-2096-z
Using smartphones to decrease substance use via self-monitoring and recovery support: study protocol for a randomized control trial
Abstract
Background: Alcohol abuse, other substance use disorders, and risk behaviors associated with the human immunodeficiency virus (HIV) represent three of the top 10 modifiable causes of mortality in the US. Despite evidence that continuing care is effective in sustaining recovery from substance use disorders and associated behaviors, patients rarely receive it. Smartphone applications (apps) have been effective in delivering continuing care to patients almost anywhere and anytime. This study tests the effectiveness of two components of such apps: ongoing self-monitoring through Ecological Momentary Assessments (EMAs) and immediate recovery support through Ecological Momentary Interventions (EMIs).
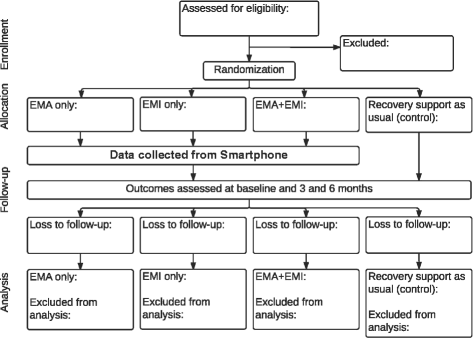
Methods/design: The target population, adults enrolled in substance use disorder treatment (n = 400), are being recruited from treatment centers in Chicago and randomly assigned to one of four conditions upon discharge in a 2 × 2 factorial design. Participants receive (1) EMAs only, (2) EMIs only, (3) combined EMAs + EMIs, or (4) a control condition without EMA or EMI for 6 months. People in the experimental conditions receive smartphones with the apps (EMA and/or EMI) specific to their condition. Phones alert participants in the EMA and EMA + EMI conditions at five random times per day and present participants with questions about people, places, activities, and feelings that they experienced in the past 30 min and whether these factors make them want to use substances, support their recovery, or have no impact. Those in the EMI and EMA + EMI conditions have continual access to a suite of support services. In the EMA + EMI condition, participants are prompted to use the EMI(s) when responses to the EMA(s) indicate risk. All groups have access to recovery support as usual. The primary outcome is days of abstinence from alcohol and other drugs. Secondary outcomes are number of HIV risk behaviors and whether abstinence mediates the effects of EMA, EMI, or EMA + EMI on HIV risk behaviors.
Discussion: This project will enable the field to learn more about the effects of EMAs and EMIs on substance use disorders and HIV risk behaviors, an understanding that could potentially make treatment and recovery more effective and more widely accessible.
Trial registration: ClinicalTrials.gov, ID: NCT02132481 . Registered on 5 May 2014.
Keywords: Ecological momentary assessment; Ecological momentary intervention; Recovery support; Smartphone; Substance use disorder; Technology; eHealth; mHealth.
Conflict of interest statement
Ethics approval and consent to participate
Participation was voluntary. The study received approval from the Chestnut Health Systems’ Institutional Review Board (IRB Study No. 1091-0114) and is registered at Clinical Trials.gov (NCT02132481). Chestnut’s IRB acted as a Centralized Ethics Committee for the two substance use treatment agencies from which participants were recruited. The study complies with the relevant Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) and World Health Organization (WHO) Checklist (see Additional file 1).
Consent for publication
Informed written consent was received for publication of the manuscript and figures. The Consent Form is held by the authors and their institution and is available for review by the Editor-in-Chief.
Competing interests
Author Dr. Gustafson has a shareholder interest in CHESS Mobile Health, a small business that develops web-based health care technology for patients and family members. This relationship is extensively managed by the authors and the University of Wisconsin. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A randomized clinical trial of smartphone self-managed recovery support services.J Subst Abuse Treat. 2020 Oct;117:108089. doi: 10.1016/j.jsat.2020.108089. Epub 2020 Jul 21. J Subst Abuse Treat. 2020. PMID: 32811628 Free PMC article. Clinical Trial.
-
A Pilot Study to Examine the Feasibility and Potential Effectiveness of Using Smartphones to Provide Recovery Support for Adolescents.Subst Abus. 2015;36(4):486-92. doi: 10.1080/08897077.2014.970323. Epub 2014 Oct 13. Subst Abus. 2015. PMID: 25310057 Free PMC article.
-
Using ecological momentary assessments to predict relapse after adult substance use treatment.Addict Behav. 2018 Jul;82:72-78. doi: 10.1016/j.addbeh.2018.02.025. Epub 2018 Feb 23. Addict Behav. 2018. PMID: 29499393 Free PMC article. Clinical Trial.
-
From Ecological Momentary Assessment (EMA) to Ecological Momentary Intervention (EMI): Past and Future Directions for Ambulatory Assessment and Interventions in Eating Disorders.Curr Psychiatry Rep. 2019 Jun 4;21(7):53. doi: 10.1007/s11920-019-1046-8. Curr Psychiatry Rep. 2019. PMID: 31161276 Review.
-
Standalone Smartphone Cognitive Behavioral Therapy-Based Ecological Momentary Interventions to Increase Mental Health: Narrative Review.JMIR Mhealth Uhealth. 2020 Nov 12;8(11):e19836. doi: 10.2196/19836. JMIR Mhealth Uhealth. 2020. PMID: 33180027 Free PMC article. Review.
Cited by
-
Narrowing the Patient-Physician Gap Based on Self-Reporting and Monthly Hepatologist Feedback for Patients With Alcohol-Related Liver Disease: Interventional Pilot Study Using a Journaling Smartphone App.JMIR Form Res. 2023 Dec 19;7:e44762. doi: 10.2196/44762. JMIR Form Res. 2023. PMID: 38113066 Free PMC article.
-
Methodological Challenges in Randomized Controlled Trials on Smartphone-Based Treatment in Psychiatry: Systematic Review.J Med Internet Res. 2019 Oct 27;21(10):e15362. doi: 10.2196/15362. J Med Internet Res. 2019. PMID: 31663859 Free PMC article.
-
Before and after: craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder.Psychopharmacology (Berl). 2018 Sep;235(9):2713-2723. doi: 10.1007/s00213-018-4966-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980821 Free PMC article.
-
Time-varying effect modeling with intensive longitudinal data: Examining dynamic links among craving, affect, self-efficacy and substance use during addiction recovery.Addiction. 2023 Nov;118(11):2220-2232. doi: 10.1111/add.16284. Epub 2023 Jul 7. Addiction. 2023. PMID: 37416972 Free PMC article.
-
Affect-laden risk profiles derived from two days of EMA predict substance use and quality of life three- and six-months after SUD treatment.J Subst Use Addict Treat. 2025 Mar;170:209613. doi: 10.1016/j.josat.2024.209613. Epub 2024 Dec 21. J Subst Use Addict Treat. 2025. PMID: 39716517 No abstract available.
References
-
- Kirschstein R. Disease-specific estimates of direct and indirect costs of illness and NIH support: fiscal year 2000 update. Bethesda: Department of Health and Human Services, National Institutes of Health, Office of the Director; 2000.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous