Conformational characterization of nerve growth factor-β reveals that its regulatory pro-part domain stabilizes three loop regions in its mature part
- PMID: 28798232
- PMCID: PMC5633128
- DOI: 10.1074/jbc.M117.803320
Conformational characterization of nerve growth factor-β reveals that its regulatory pro-part domain stabilizes three loop regions in its mature part
Abstract
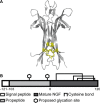
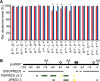
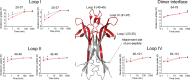
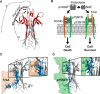
Nerve growth factor-β (NGF) is essential for the correct development of the nervous system. NGF exists in both a mature form and a pro-form (proNGF). The two forms have opposing effects on neurons: NGF induces proliferation, whereas proNGF induces apoptosis via binding to a receptor complex of the common neurotrophin receptor (p75NTR) and sortilin. The overexpression of both proNGF and sortilin has been associated with several neurodegenerative diseases. Insights into the conformational differences between proNGF and NGF are central to a better understanding of the opposing mechanisms of action of NGF and proNGF on neurons. However, whereas the structure of NGF has been determined by X-ray crystallography, the structural details for proNGF remain elusive. Here, using a sensitive MS-based analytical method to measure the hydrogen/deuterium exchange of proteins in solution, we analyzed the conformational properties of proNGF and NGF. We detected the presence of a localized higher-order structure motif in the pro-part of proNGF. Furthermore, by comparing the hydrogen/deuterium exchange in the mature part of NGF and proNGF, we found that the presence of the pro-part in proNGF causes a structural stabilization of three loop regions in the mature part, possibly through a direct molecular interaction. Moreover, using tandem MS analyses, we identified two N-linked and two O-linked glycosylations in the pro-part of proNGF. These results advance our knowledge of the conformational properties of proNGF and NGF and help provide a rationale for the diverse biological effects of NGF and proNGF at the molecular level.
Keywords: glycoprotein; hydrogen exchange mass spectrometry; hydrogen/deuterium exchange; intrinsically disordered protein; neurodegeneration; neurodegenerative disease; neurotrophin; protein conformation; protein structure.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Chao M. V. (2003) Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 - PubMed
-
- Jansen P., Giehl K., Nyengaard J. R., Teng K., Lioubinski O., Sjoegaard S. S., Breiderhoff T., Gotthardt M., Lin F., Eilers A., Petersen C. M., Lewin G. R., Hempstead B. L., Willnow T. E., and Nykjaer A. (2007) Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat. Neurosci. 10, 1449–1457 - PubMed
-
- Nykjaer A., Willnow T. E., and Petersen C. M. (2005) p75NTR: Live or let die. Curr. Opin. Neurobiol. 15, 49–57 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

