Molecular Organization Induced Anisotropic Properties of Perylene - Silica Hybrid Nanoparticles
- PMID: 28798295
- PMCID: PMC5552683
- DOI: 10.1038/s41598-017-07892-4
Molecular Organization Induced Anisotropic Properties of Perylene - Silica Hybrid Nanoparticles
Abstract
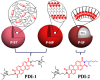
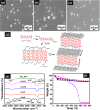
Optically active silica nanoparticles are interesting owing to high stability and easy accessibility. Unlike previous reports on dye loaded silica particles, here we address an important question on how optical properties are dependent on the aggregation-induced segregation of perylene molecules inside and outside the silica nanoparticles. Three differentially functionalized fluorescent perylene - silica hybrid nanoparticles are prepared from appropriate ratios of perylene derivatives and tetraethyl orthosilicate (TEOS) and investigated the structure property correlation (P-ST, P-NP and P-SF). The particles differ from each other on the distribution, organization and intermolecular interaction of perylene inside or outside the silica matrix. Structure and morphology of all hybrid nanoparticles were characterized using a range of techniques such as electron microscope, optical spectroscopic measurements and thermal analysis. The organizations of perylene in three different silica nanoparticles were explored using steady-state fluorescence, fluorescence anisotropy, lifetime measurements and solid state polarized spectroscopic studies. The interactions and changes in optical properties of the silica nanoparticles in presence of different amines were tested and quantified both in solution and in vapor phase using fluorescence quenching studies. The synthesized materials can be regenerated after washing with water and reused for sensing of amines.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
 ), P-SF (
), P-SF ( ), and P-NP (
), and P-NP ( ) particles in air.
) particles in air.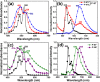
 ), P-ST2(
), P-ST2( ), P-SF (
), P-SF ( ) dispersed in THF and P-NP (
) dispersed in THF and P-NP ( ) dispersed in water. Excitation wavelength was 350 nm.
) dispersed in water. Excitation wavelength was 350 nm.
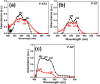
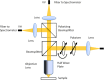
 ) to the polarizer.
) to the polarizer.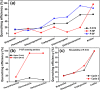
References
-
- Feng W, Wee Beng T, Yong Z, Xianping F, Minquan W. Luminescent nanomaterials for biological labelling. Nanotechnology. 2006;17:R1–R13. doi: 10.1088/0957-4484/17/1/R01. - DOI
-
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 1564–1579 (2004). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

