Luciferase Expression Allows Bioluminescence Imaging But Imposes Limitations on the Orthotopic Mouse (4T1) Model of Breast Cancer
- PMID: 28798322
- PMCID: PMC5552689
- DOI: 10.1038/s41598-017-07851-z
Luciferase Expression Allows Bioluminescence Imaging But Imposes Limitations on the Orthotopic Mouse (4T1) Model of Breast Cancer
Abstract
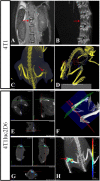
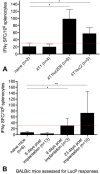
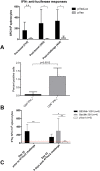
Implantation of reporter-labeled tumor cells in an immunocompetent host involves a risk of their immune elimination. We have studied this effect in a mouse model of breast cancer after the orthotopic implantation of mammary gland adenocarcinoma 4T1 cells genetically labelled with luciferase (Luc). Mice were implanted with 4T1 cells and two derivative Luc-expressing clones 4T1luc2 and 4T1luc2D6 exhibiting equal in vitro growth rates. In vivo, the daughter 4T1luc2 clone exhibited nearly the same, and 4T1luc2D6, a lower growth rate than the parental cells. The metastatic potential of 4T1 variants was assessed by magnetic resonance, bioluminescent imaging, micro-computed tomography, and densitometry which detected 100-μm metastases in multiple organs and bones at the early stage of their development. After 3-4 weeks, 4T1 generated 11.4 ± 2.1, 4T1luc2D6, 4.5 ± 0.6; and 4T1luc2, <1 metastases per mouse, locations restricted to lungs and regional lymph nodes. Mice bearing Luc-expressing tumors developed IFN-γ response to the dominant CTL epitope of Luc. Induced by intradermal DNA-immunization, such response protected mice from the establishment of 4T1luc2-tumors. Our data show that natural or induced cellular response against the reporter restricts growth and metastatic activity of the reporter-labelled tumor cells. Such cells represent a powerful instrument for improving immunization technique for cancer vaccine applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10(3):506–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

