Isoflurane produces antidepressant effects and induces TrkB signaling in rodents
- PMID: 28798343
- PMCID: PMC5552878
- DOI: 10.1038/s41598-017-08166-9
Isoflurane produces antidepressant effects and induces TrkB signaling in rodents
Abstract
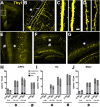
A brief burst-suppressing isoflurane anesthesia has been shown to rapidly alleviate symptoms of depression in a subset of patients, but the neurobiological basis of these observations remains obscure. We show that a single isoflurane anesthesia produces antidepressant-like behavioural effects in the learned helplessness paradigm and regulates molecular events implicated in the mechanism of action of rapid-acting antidepressant ketamine: activation of brain-derived neurotrophic factor (BDNF) receptor TrkB, facilitation of mammalian target of rapamycin (mTOR) signaling pathway and inhibition of glycogen synthase kinase 3β (GSK3β). Moreover, isoflurane affected neuronal plasticity by facilitating long-term potentiation in the hippocampus. We also found that isoflurane increased activity of the parvalbumin interneurons, and facilitated GABAergic transmission in wild type mice but not in transgenic mice with reduced TrkB expression in parvalbumin interneurons. Our findings strengthen the role of TrkB signaling in the antidepressant responses and encourage further evaluation of isoflurane as a rapid-acting antidepressant devoid of the psychotomimetic effects and abuse potential of ketamine.
Conflict of interest statement
L.K. is a paid employee in Neurotar Ltd.
Figures
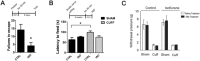




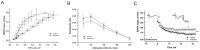

References
-
- Olesen J, et al. The economic cost of brain disorders in Europe. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2012;19:155–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

