3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair
- PMID: 28798376
- PMCID: PMC5552682
- DOI: 10.1038/s41598-017-08412-0
3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair
Abstract
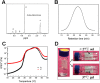
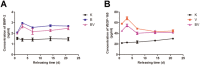
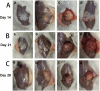
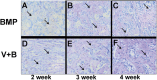
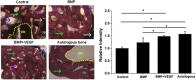
It is a great challenge to prepare "functional artificial bone" for the repair of large segmental defect, especially in weight-bearing bones. In this study, bioactive HA/PCL composite scaffolds that possess anatomical structure as autogenous bone were fabricated by CT-guided fused deposition modeling technique. The scaffolds can provide mechanical support and possess osteoconduction property. Then the VEGF-165/BMP-2 loaded hydrogel was filled into biomimetic artificial bone spatially to introduce osteoinduction and angioinduction ability via sustained release of these cytokines. It has been revealed that the cytokine-loaded hydrogel possessed good biodegradability and could release the VEGF-165/BMP-2 sustainedly and steadily. The synergistic effect of these two cytokines showed significant stimulation on the osteogenic gene expresssion of osteoblast in vitro and ectopic ossification in vivo. The scaffolds were then implanted into the rabbit tibial defect sites (1.2 cm) for bone regeneration for 12 weeks, indicating the best repair of defect in vivo, which was superior to the pure hydrogel/scaffolds or one-cytokine loaded hydrogel/scaffolds and close to autogenous bone graft. The strategy to construct an "anatomy-structure-function" trinity system as functional artificial bone shows great potential in replacing autogenous bone graft and applying in large bone defect repair clinically in future.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

