HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system
- PMID: 28798420
- PMCID: PMC5552820
- DOI: 10.1038/s41598-017-05245-9
HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system
Abstract
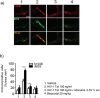
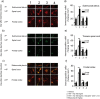
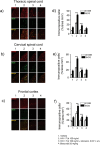
Despite the effectiveness of combined anti-retroviral therapy, human immunodeficiency virus (HIV) infected-patients frequently report diarrhea and neuropsychological deficits. It is claimed that the viral HIV-1 Trans activating factor (HIV-1 Tat) protein is responsible for both diarrhea and neurotoxic effects, but the underlying mechanisms are not known. We hypothesize that colonic application of HIV-1 Tat activates glial cells of the enteric nervous system (EGCs), leading to a neuroinflammatory response able to propagate to the central nervous system. We demonstrated that HIV-1 Tat-induced diarrhea was associated with a significant activation of glial cells within the colonic wall, the spinal cord and the frontal cortex, and caused a consistent impairment of the cognitive performances. The inhibition of glial cells activity by lidocaine, completely abolished the above-described effects. These observations point out the role of glial cells as putative effectors in HIV-1 Tat-associated gastrointestinal and neurological manifestations and key regulators of gut-brain signaling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

