Vestibular Modulation of Sympathetic Nerve Activity to Muscle and Skin in Humans
- PMID: 28798718
- PMCID: PMC5526846
- DOI: 10.3389/fneur.2017.00334
Vestibular Modulation of Sympathetic Nerve Activity to Muscle and Skin in Humans
Abstract
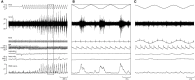
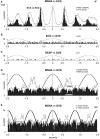
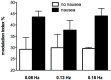
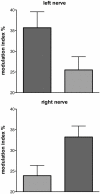
We review the existence of vestibulosympathetic reflexes in humans. While several methods to activate the human vestibular apparatus have been used, galvanic vestibular stimulation (GVS) is a means of selectively modulating vestibular afferent activity via electrodes over the mastoid processes, causing robust vestibular illusions of side-to-side movement. Sinusoidal GVS (sGVS) causes partial entrainment of sympathetic outflow to muscle and skin. Modulation of muscle sympathetic nerve activity (MSNA) from vestibular inputs competes with baroreceptor inputs, with stronger temporal coupling to the vestibular stimulus being observed at frequencies remote from the cardiac frequency; "super entrainment" was observed in some individuals. Low-frequency (<0.2 Hz) sGVS revealed two peaks of modulation per cycle, with bilateral recordings of MSNA or skin sympathetic nerve activity, providing evidence of lateralization of sympathetic outflow during vestibular stimulation. However, it should be noted that GVS influences the firing of afferents from the entire vestibular apparatus, including the semicircular canals. To identify the specific source of vestibular input responsible for the generation of vestibulosympathetic reflexes, we used low-frequency (<0.2 Hz) sinusoidal linear acceleration of seated or supine subjects to, respectively, target the utricular or saccular components of the otoliths. While others had discounted the semicircular canals, we showed that the contributions of the utricle and saccule to the vestibular modulation of MSNA are very similar. Moreover, that modulation of MSNA occurs at accelerations well below levels at which subjects are able to perceive any motion indicates that, like vestibulospinal control of posture, the vestibular system contributes to the control of blood pressure through potent reflexes in humans.
Keywords: galvanic vestibular stimulation; linear acceleration; muscle sympathetic nerve activity; skin sympathetic nerve activity; vestibular system; vestibulosympathetic reflexes.
Figures





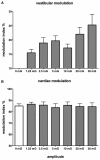
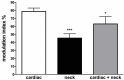
References
-
- Cannon WB. The Wisdom of the Body. New York: W. W. Norton; (1963).
-
- Jänig W, McLachlan EM. Neurobiology of the autonomic nervous system. In: Mathias CJ, Bannister SR, editors. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford: Oxford University Press; (2013). p. 21–34.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

