The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction
- PMID: 28798857
- PMCID: PMC5533495
- DOI: 10.1063/1.4991738
The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction
Abstract
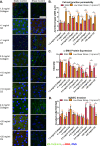
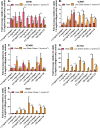
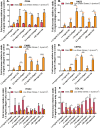
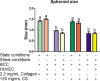
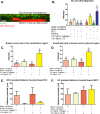
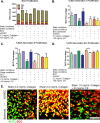
Tumor development is influenced by stromal cells in aspects including invasion, growth, angiogenesis, and metastasis. Activated fibroblasts are one group of stromal cells involved in cancer metastasis, and one source of activated fibroblasts is endothelial to mesenchymal transformation (EndMT). EndMT begins when the endothelial cells delaminate from the cell monolayer, lose cell-cell contacts, lose endothelial markers such as vascular endothelial-cadherin (VE-cadherin), gain mesenchymal markers like alpha-smooth muscle actin (α-SMA), and acquire mesenchymal cell-like properties. A three-dimensional (3D) culture microfluidic device was developed for investigating the role of steady low shear stress (1 dyne/cm2) and altered extracellular matrix (ECM) composition and stiffness on EndMT. Shear stresses resulting from fluid flow within tumor tissue are relevant to both cancer metastasis and treatment effectiveness. Low and oscillatory shear stress rates have been shown to enhance the invasion of metastatic cancer cells through specific changes in actin and tubulin remodeling. The 3D ECM within the device was composed of type I collagen and glycosaminoglycans (GAGs), hyaluronic acid and chondroitin sulfate. An increase in collagen and GAGs has been observed in the solid tumor microenvironment and has been correlated with poor prognosis in many different cancer types. In this study, it was found that ECM composition and low shear stress upregulated EndMT, including upregulation of mesenchymal-like markers (α-SMA and Snail) and downregulated endothelial marker protein and gene expression (VE-cadherin). Furthermore, this novel model was utilized to investigate the role of EndMT in breast cancer cell proliferation and migration. Cancer cell spheroids were embedded within the 3D ECM of the microfluidic device. The results using this device show for the first time that the breast cancer spheroid size is dependent on shear stress and that the cancer cell migration rate, distance, and proliferation are induced by EndMT-derived activated fibroblasts. This model can be used to explore new therapeutics in a tumor microenvironment.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
