Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models
- PMID: 28798911
- PMCID: PMC5526851
- DOI: 10.3389/fbioe.2017.00040
Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models
Abstract
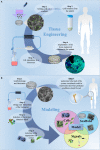
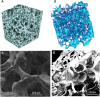
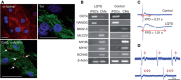
In the tissue engineering (TE) paradigm, engineering and life sciences tools are combined to develop bioartificial substitutes for organs and tissues, which can in turn be applied in regenerative medicine, pharmaceutical, diagnostic, and basic research to elucidate fundamental aspects of cell functions in vivo or to identify mechanisms involved in aging processes and disease onset and progression. The complex three-dimensional (3D) microenvironment in which cells are organized in vivo allows the interaction between different cell types and between cells and the extracellular matrix, the composition of which varies as a function of the tissue, the degree of maturation, and health conditions. In this context, 3D in vitro models can more realistically reproduce a tissue or organ than two-dimensional (2D) models. Moreover, they can overcome the limitations of animal models and reduce the need for in vivo tests, according to the "3Rs" guiding principles for a more ethical research. The design of 3D engineered tissue models is currently in its development stage, showing high potential in overcoming the limitations of already available models. However, many issues are still opened, concerning the identification of the optimal scaffold-forming materials, cell source and biofabrication technology, and the best cell culture conditions (biochemical and physical cues) to finely replicate the native tissue and the surrounding environment. In the near future, 3D tissue-engineered models are expected to become useful tools in the preliminary testing and screening of drugs and therapies and in the investigation of the molecular mechanisms underpinning disease onset and progression. In this review, the application of TE principles to the design of in vitro 3D models will be surveyed, with a focus on the strengths and weaknesses of this emerging approach. In addition, a brief overview on the development of in vitro models of healthy and pathological bone, heart, pancreas, and liver will be presented.
Keywords: bone; heart; liver; models; pancreas; three-dimensional; tissue engineering.
Figures


References
-
- Alvarez K., Nakajima H. (2009). Metallic scaffolds for bone regeneration. Materials 2, 790–832. 10.3390/ma2030790 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

