Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment
- PMID: 28798979
- PMCID: PMC11029177
- DOI: 10.1007/s00262-017-2050-7
Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment
Abstract
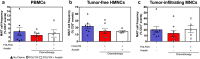
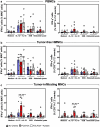
Mucosa-associated invariant T (MAIT) cells are innate-like T lymphocytes that are unusually abundant in the human liver, a common site of colorectal carcinoma (CRC) metastasis. However, whether they contribute to immune surveillance against colorectal liver metastasis (CRLM) is essentially unexplored. In addition, whether MAIT cell functions can be impacted by chemotherapy is unclear. These are important questions given MAIT cells' potent immunomodulatory and inflammatory properties. Herein, we examined the frequencies and functions of peripheral blood, healthy liver tissue, tumor-margin and tumor-infiltrating MAIT cells in 21 CRLM patients who received no chemotherapy, FOLFOX, or a combination of FOLFOX and Avastin before they underwent liver resection. We found that MAIT cells, defined as CD3ε+Vα7.2+CD161++ or CD3ε+MR1 tetramer+ cells, were present within both healthy and tumor-afflicted hepatic tissues. Paired and grouped analyses of samples revealed the physical proximity of MAIT cells to metastatic lesions to drastically influence their functional competence. Accordingly, unlike those residing in the healthy liver compartment, tumor-infiltrating MAIT cells failed to produce IFN-γ in response to a panel of TCR and cytokine receptor ligands, and tumor-margin MAIT cells were only partially active. Furthermore, chemotherapy did not account for intratumoral MAIT cell insufficiencies. Our findings demonstrate for the first time that CRLM-penetrating MAIT cells exhibit wide-ranging functional impairments, which are dictated by their physical location but not by preoperative chemotherapy. Therefore, we propose that MAIT cells may provide an attractive therapeutic target in CRC and that their ligands may be combined with chemotherapeutic agents to treat CRLM.
Keywords: Chemotherapy; Colon cancer; Immune surveillance; Liver metastasis; MAIT cells; Tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour AL, Stein KD, Sharpe KB, Brooks DD, Cowens-Alvarado RL. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin. 2015;65(6):428–455. doi: 10.3322/caac.21286. - DOI - PMC - PubMed
-
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–259. doi: 10.1097/01.sla.0000217629.94941.cf. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

