The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability
- PMID: 28799444
- PMCID: PMC6174619
- DOI: 10.1177/1352458517723718
The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability
Abstract
Background: The Multiple Sclerosis Outcome Assessments Consortium (MSOAC) was formed by the National MS Society to develop improved measures of multiple sclerosis (MS)-related disability.
Objectives: (1) To assess the current literature and available data on functional performance outcome measures (PerfOs) and (2) to determine suitability of using PerfOs to quantify MS disability in MS clinical trials.
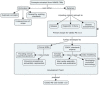
Methods: (1) Identify disability dimensions common in MS; (2) conduct a comprehensive literature review of measures for those dimensions; (3) develop an MS Clinical Data Interchange Standards Consortium (CDISC) data standard; (4) create a database of standardized, pooled clinical trial data; (5) analyze the pooled data to assess psychometric properties of candidate measures; and (6) work with regulatory agencies to use the measures as primary or secondary outcomes in MS clinical trials.
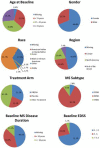
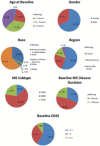
Conclusion: Considerable data exist supporting measures of the functional domains ambulation, manual dexterity, vision, and cognition. A CDISC standard for MS ( http://www.cdisc.org/therapeutic#MS ) was published, allowing pooling of clinical trial data. MSOAC member organizations contributed clinical data from 16 trials, including 14,370 subjects. Data from placebo-arm subjects are available to qualified researchers. This integrated, standardized dataset is being analyzed to support qualification of disability endpoints by regulatory agencies.
Keywords: MS disability; clinical trial database; data standards; performance outcome measures; regulatory qualification.
Conflict of interest statement
Figures



References
-
- Whitaker JN, McFarland HF, Rudge P, et al. Outcomes assessment in multiple sclerosis clinical trials: A critical analysis. Mult Scler 1995; 1(1): 37–47. - PubMed
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33(11): 1444–1452. - PubMed
-
- Rudick R, Antel J, Confavreux C, et al. Clinical outcomes assessment in multiple sclerosis. Ann Neurol 1996; 40(3): 469–479. - PubMed
-
- Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the multiple sclerosis functional composite. Neurology 2003; 61(10): 1367–1373. - PubMed
-
- Ontaneda D, LaRocca N, Coetzee T, et al. ; NMSS MSFC Task Force. Revisiting the multiple sclerosis functional composite: Proceedings from the National Multiple Sclerosis Society (NMSS) Task Force on Clinical Disability Measures. Mult Scler 2012; 18(8): 1074–1080. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

