Glycan Phosphorylases in Multi-Enzyme Synthetic Processes
- PMID: 28799504
- PMCID: PMC5688430
- DOI: 10.2174/0929866524666170811125109
Glycan Phosphorylases in Multi-Enzyme Synthetic Processes
Abstract
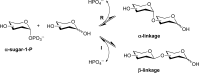
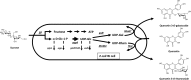
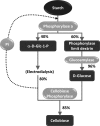
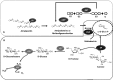
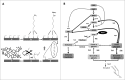
Glycoside phosphorylases catalyse the reversible synthesis of glycosidic bonds by glycosylation with concomitant release of inorganic phosphate. The equilibrium position of such reactions can render them of limited synthetic utility, unless coupled with a secondary enzymatic step where the reaction lies heavily in favour of product. This article surveys recent works on the combined use of glycan phosphorylases with other enzymes to achieve synthetically useful processes.
Keywords: Phosphorylase; biofuel; cellodextrin; disaccharide; high-value products; α-glucan.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures



Similar articles
-
Characterization of Ruminococcus albus cellodextrin phosphorylase and identification of a key phenylalanine residue for acceptor specificity and affinity to the phosphate group.FEBS J. 2013 Sep;280(18):4463-73. doi: 10.1111/febs.12408. Epub 2013 Jul 19. FEBS J. 2013. PMID: 23802549
-
Crystal Structure and Substrate Recognition of Cellobionic Acid Phosphorylase, Which Plays a Key Role in Oxidative Cellulose Degradation by Microbes.J Biol Chem. 2015 Jul 24;290(30):18281-92. doi: 10.1074/jbc.M115.664664. Epub 2015 Jun 3. J Biol Chem. 2015. PMID: 26041776 Free PMC article.
-
Recombinant production and biochemical characterization of a hyperthermostable alpha-glucan/maltodextrin phosphorylase from Pyrococcus furiosus.Archaea. 2008 Dec;2(3):169-76. doi: 10.1155/2008/549759. Archaea. 2008. PMID: 19054743 Free PMC article.
-
Enzymatic synthesis using glycoside phosphorylases.Carbohydr Res. 2015 Feb 11;403:23-37. doi: 10.1016/j.carres.2014.06.010. Epub 2014 Jun 18. Carbohydr Res. 2015. PMID: 25060838 Free PMC article. Review.
-
Carbohydrate synthesis by disaccharide phosphorylases: reactions, catalytic mechanisms and application in the glycosciences.Biotechnol J. 2010 Dec;5(12):1324-38. doi: 10.1002/biot.201000217. Biotechnol J. 2010. PMID: 21154671 Review.
Cited by
-
Recent advances in enzymatic synthesis of β-glucan and cellulose.Carbohydr Res. 2021 Oct;508:108411. doi: 10.1016/j.carres.2021.108411. Epub 2021 Jul 24. Carbohydr Res. 2021. PMID: 34392134 Free PMC article. Review.
-
Engineering cascade biocatalysis in whole cells for bottom-up synthesis of cello-oligosaccharides: flux control over three enzymatic steps enables soluble production.Microb Cell Fact. 2022 Apr 9;21(1):61. doi: 10.1186/s12934-022-01781-w. Microb Cell Fact. 2022. PMID: 35397553 Free PMC article.
-
Recent progress in synthesis of carbohydrates with sugar nucleotide-dependent glycosyltransferases.Curr Opin Chem Biol. 2021 Apr;61:81-95. doi: 10.1016/j.cbpa.2020.10.007. Epub 2020 Dec 10. Curr Opin Chem Biol. 2021. PMID: 33310623 Free PMC article. Review.
-
The structure of a GH149 β-(1 → 3) glucan phosphorylase reveals a new surface oligosaccharide binding site and additional domains that are absent in the disaccharide-specific GH94 glucose-β-(1 → 3)-glucose (laminaribiose) phosphorylase.Proteins. 2019 Oct;87(10):885-892. doi: 10.1002/prot.25745. Epub 2019 Jun 6. Proteins. 2019. PMID: 31134667 Free PMC article.
-
Preparative and Kinetic Analysis of β-1,4- and β-1,3-Glucan Phosphorylases Informs Access to Human Milk Oligosaccharide Fragments and Analogues Thereof.Chembiochem. 2020 Apr 1;21(7):1043-1049. doi: 10.1002/cbic.201900440. Epub 2019 Dec 30. Chembiochem. 2020. PMID: 31657512 Free PMC article.
References
-
- Field R.A. Glycobiology: Challenging reaction equilibria. Nat. Chem. Biol. 2011;7:658–659. - PubMed
-
- Puchart V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015;33:261–276. - PubMed
-
- Nakai H., Kitaoka M., Svensson B., Ohtsubo K.I. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013;17:301–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

