Phosphate-Linked Silibinin Dimers (PLSd): New Promising Modified Metabolites
- PMID: 28800072
- PMCID: PMC6152259
- DOI: 10.3390/molecules22081323
Phosphate-Linked Silibinin Dimers (PLSd): New Promising Modified Metabolites
Abstract
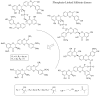
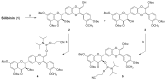
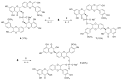
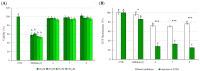
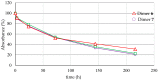
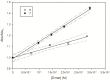
By exploiting the regioselective protection of the hydroxyl groups of silibinin along with the well-known phosphoramidite chemistry, we have developed an efficient strategy for the synthesis of new silibinin-modified species, which we have named Phosphate-Linked Silibinin Dimers (PLSd), in which the monomer units are linked by phosphodiester bonds. The antioxidant abilities of the new PLSd were estimated on HepG2 cells using DPPH free radical scavenging and xanthine/xanthine oxidase assays. The new phosphate-metabolites showed a higher anti-oxidant activity than the silibinin, as well as very low toxicity. The ability to scavenge reactive oxygen species (ROS) such as singlet oxygen () and hydroxyl radical () reveals that the two dimers are able to scavenge about two times more effectively than silibinin. Finally, solubility studies have shown that the PLSd present good water solubility (more than 20 mg·L-1) under circumneutral pH values, whereas the silibinin was found to be very poorly soluble (less than 0.4 mg·L-1) and not stable under alkaline conditions. Together, the above promising results warrant further investigation of the future potential of the PLSd as anti-oxidant metabolites within the large synthetic polyphenols field.
Keywords: oligoflavonoids; phosphodiester; radical scavengers; reactive oxygen species (ROS); silibinin; xanthine/xanthine oxidase assay.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Bernini R., Fernanda C., Barontini M., Tofani D., Balducci V., Gambacorta A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidant structural analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012;60:7408–7416. doi: 10.1021/jf301131a. - DOI - PubMed
-
- Barontini M., Bernini R., Carastro I., Gentili P., Romani A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014;38:809–816. doi: 10.1039/C3NJ01180A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

